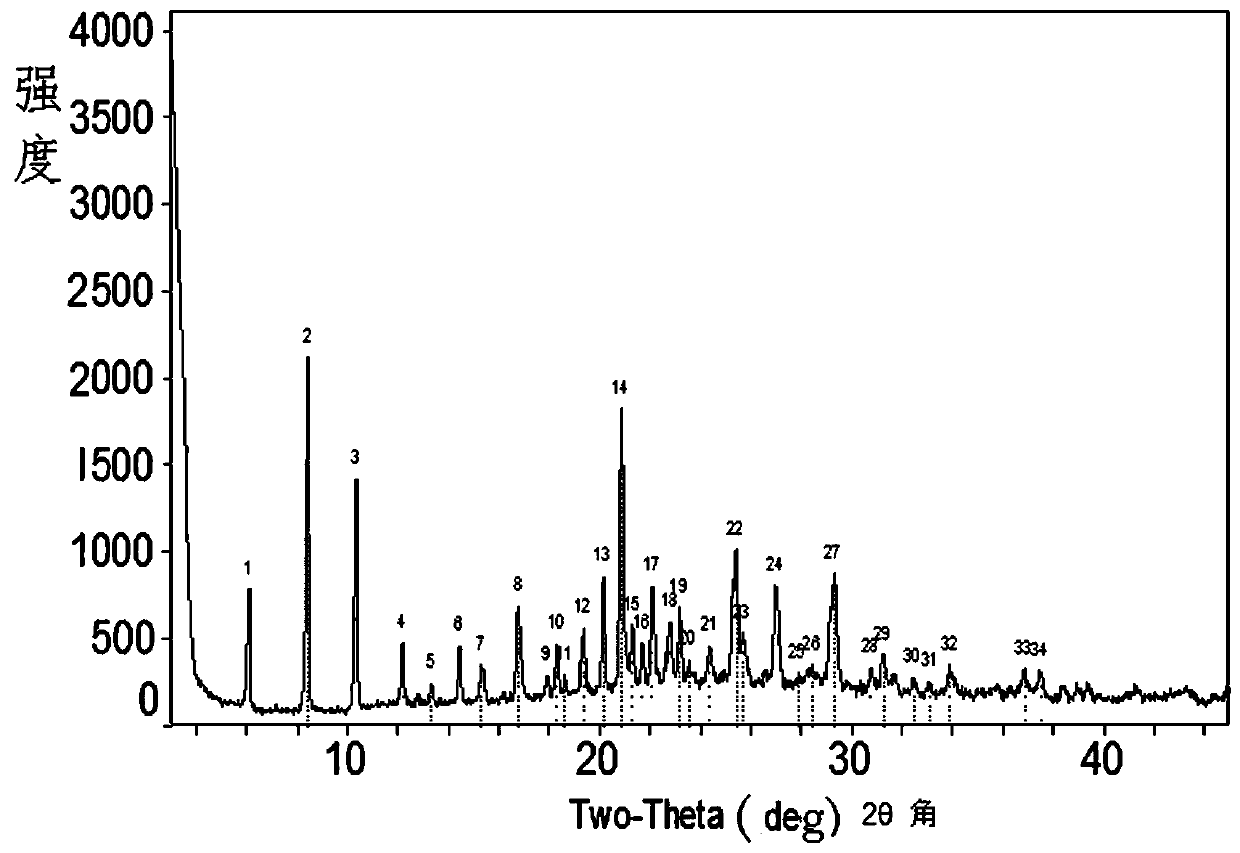
Benzoic acid compounds and preparation method and application thereof
A technology for benzoic acids and compounds, applied in the field of medicinal chemistry, can solve problems such as increased mortality and affect the recovery of diseases, and achieve the effects of being easy to prepare, suitable for large-scale production, and reducing brain cell damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of compound 8
[0041]
[0042] Starting material 6 (2.0g, 9.39mmol) and NBS (N-bromosuccinimide) (1.84g, 1.5mmol), AIBN (azobisisobutyronitrile) (154mg, 0.94mmol) were added to tetrachloride Carbon (30mL). The reaction mixture was refluxed for 2h, cooled to room temperature and filtered. The solvent was evaporated to obtain compound 7. Add water (10mL) and reflux for 1h, cool, add EtOAc (200mL) for extraction, the organic phase is washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent is evaporated to obtain compound 8 (2.05g, 95%) without purification used directly in the next reaction.
Embodiment 2
[0043] Embodiment 2: the preparation of compound 10
[0044]
[0045] Magnesium bars (0.63g, 26.22mmol) were added to a three-necked flask, dry THF (tetrahydrofuran) (20mL) and a grain of iodine were added, and 4-bromo-1-butene (2.95 g, 21.83mmol) THF (2mL) solution to initiate the reaction, after the dropwise addition was completed, the reaction was refluxed for 2h. Compound 8 (1.0g, 4.37mmol) was dissolved in THF (10mL), under the protection of nitrogen, the newly prepared Grignard reagent was added dropwise at 0°C, after the addition was completed, it was raised to room temperature for 2h, and 1N HCl was added dropwise at 0°C to quench extinguished, evaporated THF, added DCM to dissolve, added 1N HCl to adjust the pH to 2, reacted at room temperature overnight, added EtOAc (100mL), separated the organic phase, dried over anhydrous sodium sulfate, filtered, evaporated the solvent, and the residue was used on a silica gel column Purification gave compound 9 (325 mg, 28%) ...
Embodiment 3
[0047] Embodiment 3: the preparation of compound 12
[0048]
[0049] Add magnesium strips (125mg, 5.22mmol) into a three-necked flask, add dry THF (5mL) and a grain of iodine, and gradually add (4-bromobutoxy)(tert-butyl) dropwise under nitrogen protection and heating. A solution of dimethylsilane (1.16g, 4.35mmol) in THF (4mL) initiated the reaction, after the dropwise addition was completed, the reaction was refluxed for 2h to obtain the Grignard reagent. Compound 8 (200mg, 0.87mmol) was dissolved in THF (5mL), under the protection of nitrogen, the newly prepared Grignard reagent was added dropwise at 0°C, after the addition was completed, it was raised to room temperature for 2h, and quenched by adding 1N HCl dropwise at 0°C , evaporate THF, add DCM (50mL) to dissolve, add 1N HCl to adjust the pH to 2, react at room temperature overnight, extract, separate the organic phase, dry over anhydrous sodium sulfate, filter, evaporate the solvent, and the residue is subjected t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com