Preparation method of biodegradable block copolymer
A block copolymer and biodegradation technology, applied in the field of preparation of biodegradable block copolymers, can solve the problems of cumbersome synthesis process, high production cost, increased activity, etc., and achieve simple operation, improved mechanical properties, and improved activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
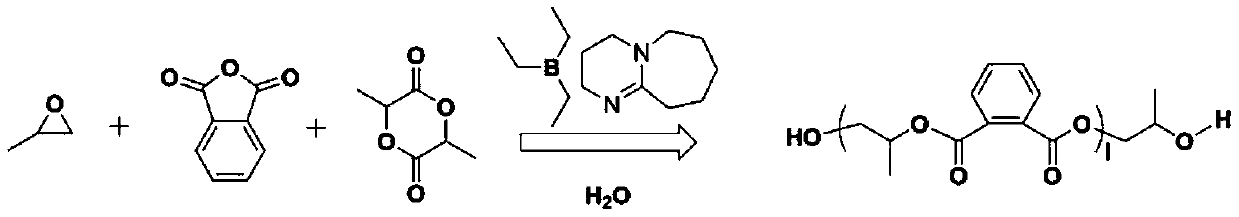
[0034] The invention provides a method for preparing a biodegradable block copolymer. By using a hydroxyl terminal compound as an initiator, a non-metallic Lewis acid and a Lewis base catalyst are used to synergistically catalyze epoxy monomers, acid anhydride monomers and lactones Biodegradable block copolymers were prepared by one-step polymerization of similar monomers. The concrete realization steps of this technical scheme are as follows:
[0035] (1) Under anaerobic conditions, add epoxy monomers, lactone monomers, and acid anhydride monomers into the reactor according to a certain ratio to obtain a monomer mixture; determine according to the needs of the target block copolymer The mixing ratio. In some embodiments, the molar ratio of the anhydride monomer to the epoxy monomer is 50:500 to 200:500; the molar ratio of the lactone monomer to the epoxy monomer is 50:500~200:500.
[0036] (2) mixing the monomer mixture described in step (1) with the initiator to obtain a ...
Embodiment 1
[0069] A kind of preparation method of degradable triblock copolymer comprises the following steps:
[0070] (1) Under anhydrous and oxygen-free conditions, mix ε-caprolactone, 1,2-cyclohexanedioic anhydride, 1,2-epoxybutane, and deionized water in a molar ratio of 50:100:500:10 Take 0.5ml, 1.29g, 3.6mL, and 15μL respectively, and add them to a 10mL reaction kettle in turn, and then add the catalyst triethylboron (85μL, 1mol / L) and 1,5,7-triazide bicyclo (4.4.0 ) dec-5-ene (60mg) was added to the reaction system in a molar ratio of 1:5, stirred and reacted at 100°C for 48h, then the reactor was cooled to room temperature, and air was introduced to terminate the reaction. The reaction solution was yellow and viscous thick.
[0071] (2) Dissolve the reaction solution in step (1) in 10 mL of dichloromethane, slowly drop it into cold methanol solution to precipitate a polymer, filter and separate to obtain a white polymer, and dry it in a vacuum oven at 40°C to constant weight ....
Embodiment 2
[0080] A kind of preparation method of degradable triblock copolymer comprises the following steps:
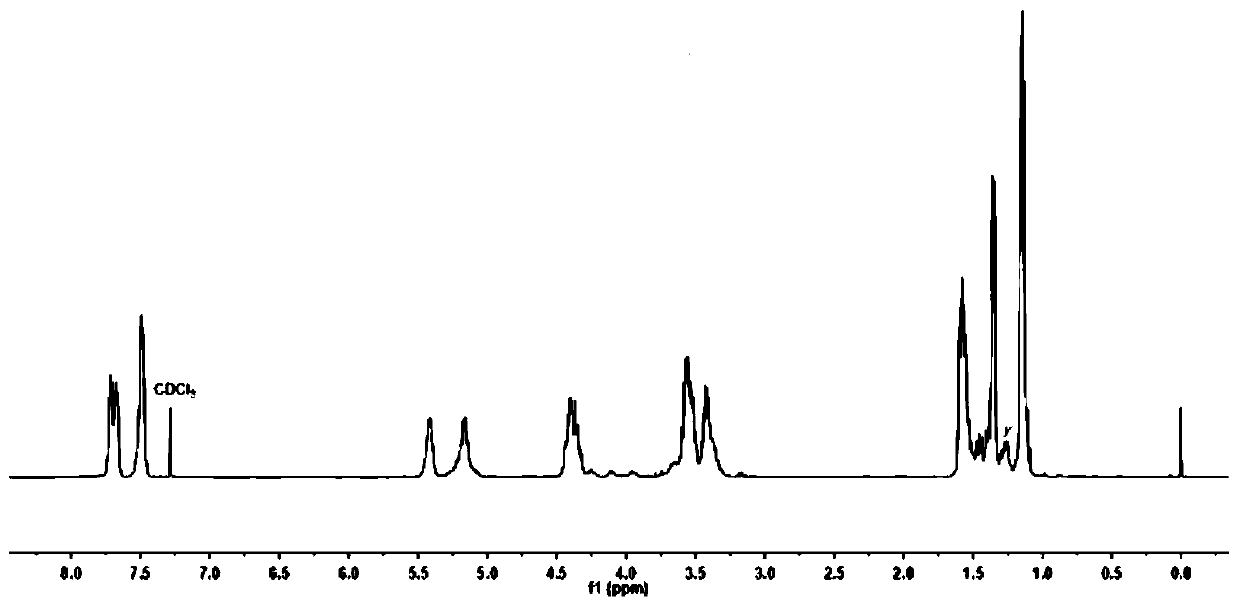
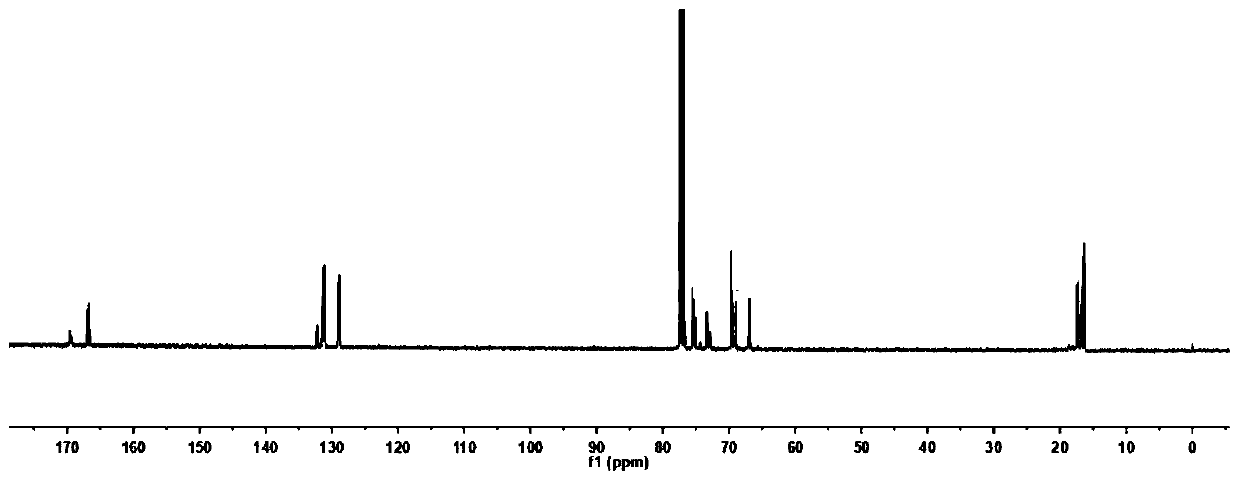
[0081] (1) Under anhydrous and oxygen-free conditions, take 1.20g, 3mL, and 1.5μL of lactide, propylene oxide, and deionized water in a molar ratio of 100:500:1, respectively, and add them to a 10mL reaction kettle in sequence. Add the catalyst triethylboron (170 μL, 1mol / L) and 7-methyl-1,5,7-triazabicyclo (4.4.0) dec-5-ene (12 μL) in a molar ratio of 2:1 into the reaction system, stirred and reacted at 40°C for 72 hours, then cooled the reactor to room temperature, and added dilute hydrochloric acid to terminate the reaction, the reaction solution was yellow and relatively viscous.
[0082] (2) Dissolve the reaction solution in step (1) in 10 mL of dichloromethane, slowly drop it into cold methanol solution to precipitate a polymer, filter and separate to obtain a white polymer, and dry it in a vacuum oven at 40°C to constant weight .
[0083] (3) H NMR spectrum and C NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com