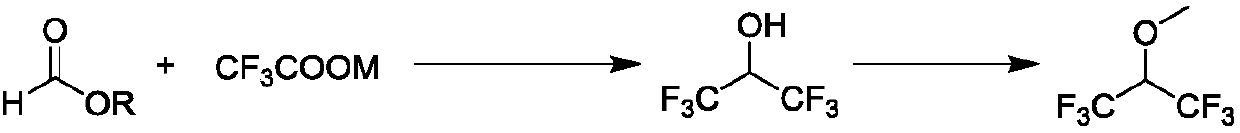
Preparation method of hexafluoroisopropyl methyl ether
A technology of hexafluoroisopropyl methyl ether and hexafluoroisopropanol, which is applied in the field of preparation of hexafluoroisopropyl methyl ether, achieves high reaction yield, mild and controllable reaction conditions, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 47.6 g (0.25 mol) of cuprous iodide, 51 g (0.37 mol) of sodium trifluoroacetate, and 18.5 g (0.25 mol) of ethyl formate into a 250 mL three-necked flask equipped with a constant pressure dropping funnel, a thermometer and a reflux condenser. ) and 100ml DMF, drain the air in the system, and replace it twice with nitrogen, and heated and stirred to 170°C under the protection of nitrogen, and stirred at 170°C for 2 hours, quickly added 2ml 1M HCl solution, and continued at 170°C React at ℃ for 4 hours, and monitor the reaction process with gas chromatography, stop heating after the reaction is complete, let the reaction solution stand for cooling, filter, add 22.5g (0.25mol) of dimethyl carbonate to the filtrate without purification, 10g (0.25mol) of sodium hydroxide ), magnetically stirred and heated to 90°C for reflux for 4 hours, monitored the reaction process with gas chromatography, after the reaction was complete, left to cool, distilled, and got the fraction wit...
Embodiment 2
[0055]Add 47.6 g (0.25 mol) of cuprous iodide, 48.2 g (0.37 mol) of ammonium trifluoroacetate, and 18.5 g (0.25 mol) and 100ml DMF, drain the air in the system, and replace it twice with nitrogen, and heat and stir to 170°C under the protection of nitrogen, and stir at 170°C for 2 hours, quickly add 2ml 1M HCl solution, continue to React at 170°C for 4 hours, and monitor the reaction process with gas chromatography. After the reaction is complete, stop heating, and the reaction solution is left to cool, filtered, and the filtrate is added without purification. 22.5g (0.25mol) of dimethyl carbonate, 10g (0.25mol) of sodium hydroxide mol), magnetically stirred and heated to 90°C for reflux for 4 hours, monitored the reaction process with gas chromatography, after the reaction was complete, left to cool, distilled, and got the fraction with a boiling point of 50-51°C to obtain 42.8g of product.
[0056] After testing, the product was 1,1,1,3,3,3-hexafluoroisopropanol methyl ether...
Embodiment 3
[0058] Add 47.6 g (0.25 mol) of cuprous iodide, 56.24 g (0.37 mol) of potassium trifluoroacetate, and 18.5 g (0.25 mol) and 100ml DMF, drain the air in the system, and replace it twice with nitrogen, and heat and stir to 170°C under the protection of nitrogen, and stir at 170°C for 2 hours, quickly add 2ml 1M HCl solution, continue to React at 170°C for 4 hours, and monitor the reaction process with gas chromatography. After the reaction is complete, stop heating, and the reaction solution is left to cool, filtered, and the filtrate is added without purification. 22.5g (0.25mol) of dimethyl carbonate, 10g (0.25mol) of sodium hydroxide mol), heated to 90°C with magnetic stirring and refluxed for 4 hours, monitored the reaction process with gas chromatography, after the reaction was complete, left to cool, distilled, and took the fraction with a boiling point of 50-51°C to obtain 42.2g of product.
[0059] After testing, the product was 1,1,1,3,3,3-hexafluoroisopropanol methyl e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com