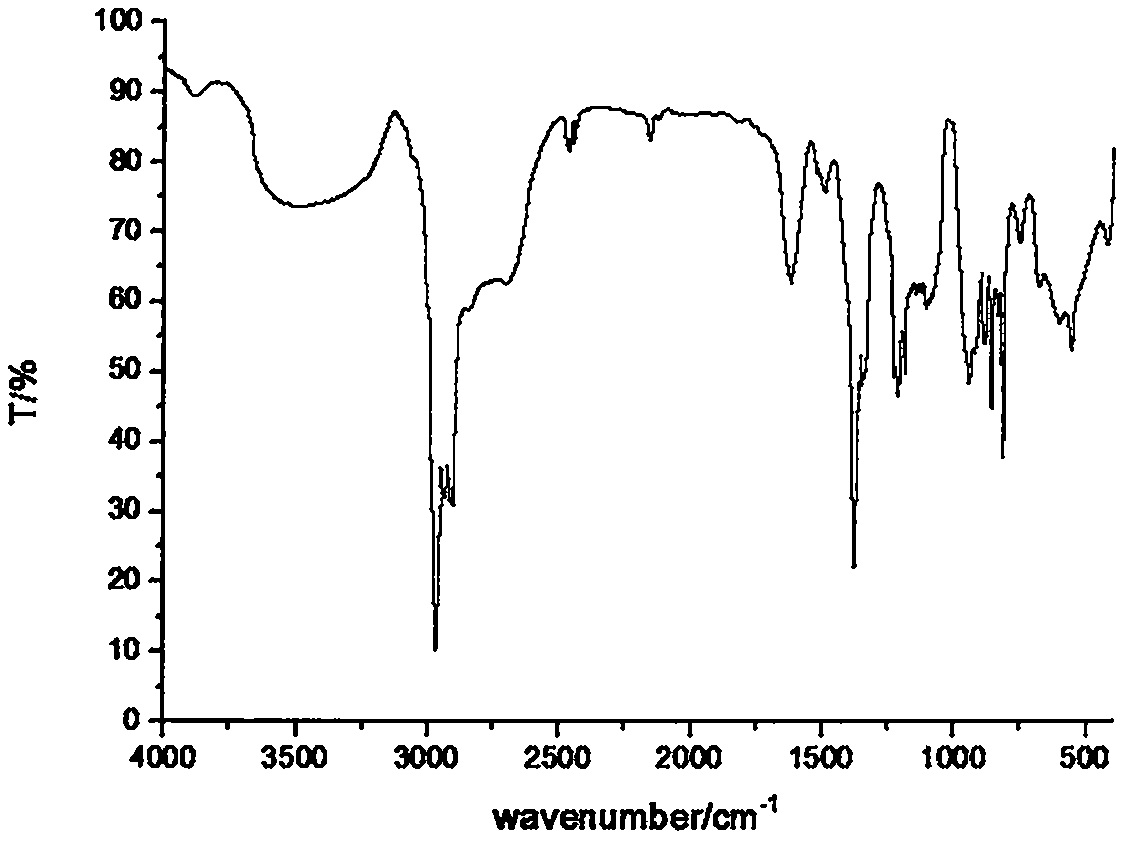
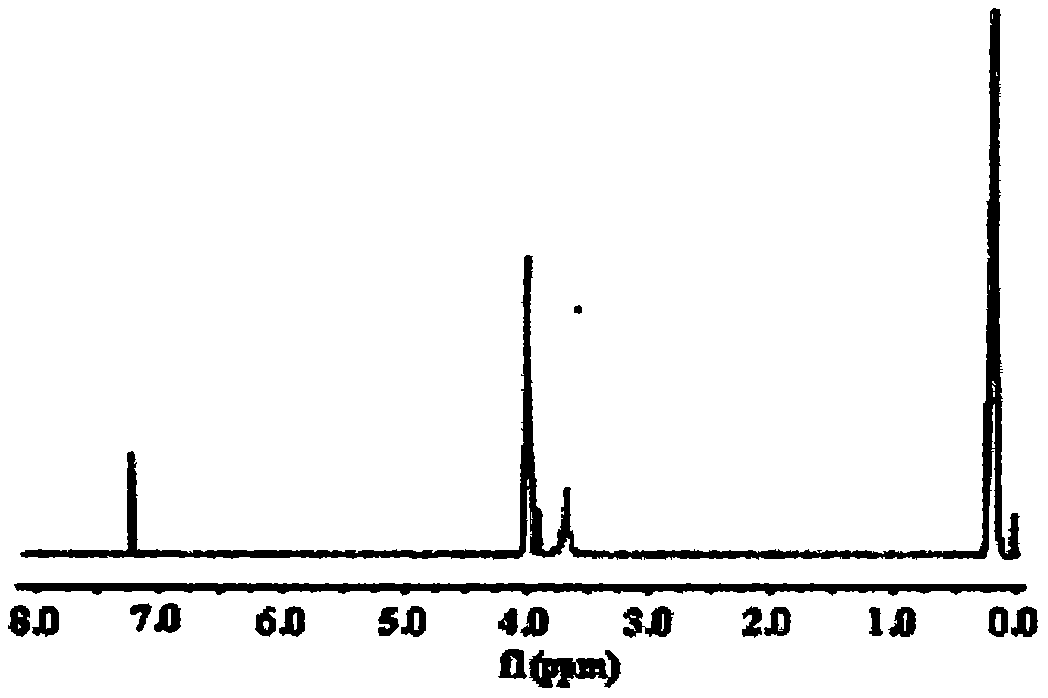
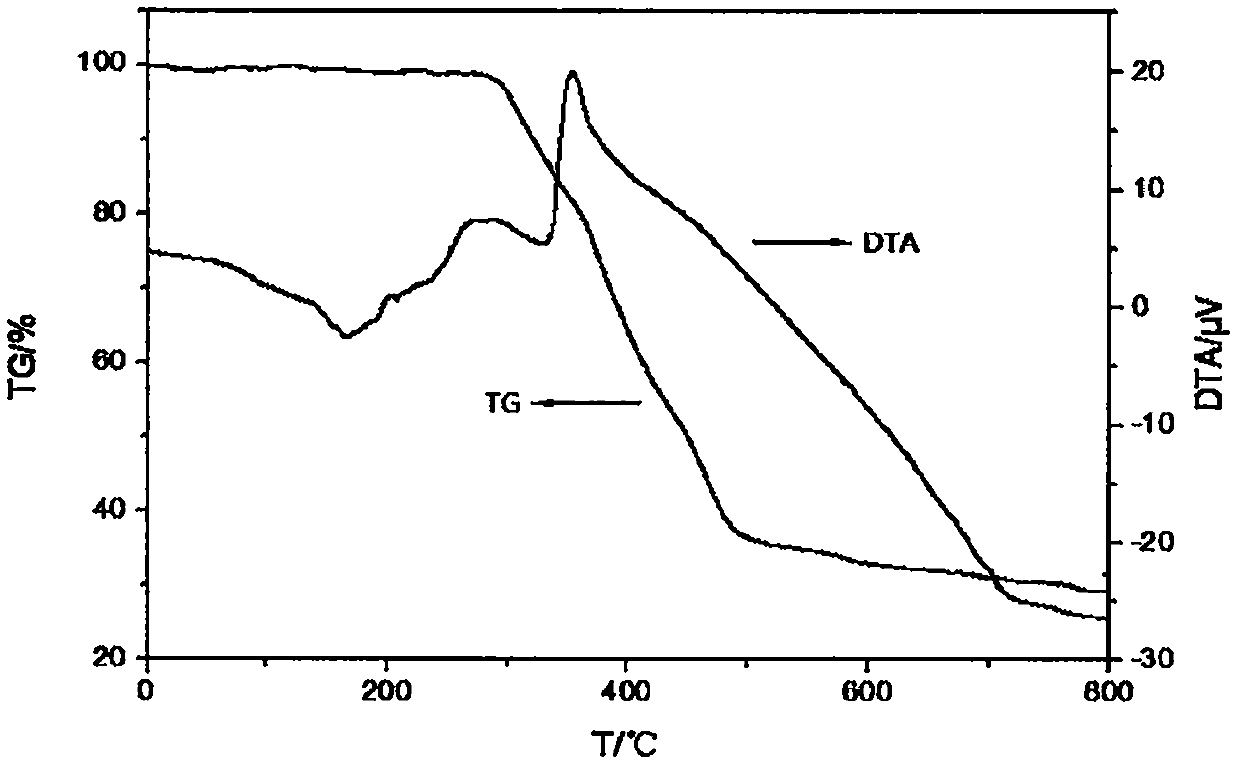
Fire retardant namely tri(dimethyl silicon phosphonyl heterocyclic methylene) thiophosphate compound and preparation method thereof
A technology of dimethylsilylphosphonyl heterocyclic methylene and flame retardant thiophosphoric acid tris, which is applied to the flame retardant thiophosphoric acid tris (dimethyl silylphosphono heterocyclic methylene) ester compound and the same In the field of preparation, it can solve problems such as secondary hazards to people's life safety, and achieve the effects of easy large-scale transformation and production, high decomposition temperature, and low equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, use nitrogen to exhaust the air in the bottle, and at 20°C, add 29.4g ( 0.15mol) trimethylol phosphine oxide cyclic ester of dimethyl silicate and 90ml of diethylene glycol dimethyl ether, after the trimethylol phosphine oxide cyclic ester of dimethyl silicate is completely dissolved and dispersed, add dropwise 8.47g (0.05mol) of phosphorus trichloride, control the dropwise addition process not to exceed 60°C, after the dropwise addition, the temperature is raised to 120°C, and the heat preservation reaction is carried out for 10h, so that the hydrogen chloride is released completely, and the solvent is distilled off under reduced pressure to obtain a turbid viscous liquid; The crude product was poured into 100 ml of ice water and stirred rapidly for 30 min, filtered with s...
Embodiment 2
[0026] Example 2 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, use nitrogen to exhaust the air in the bottle, and at 20°C, add 30.38g ( 0.155mol) trimethylol phosphine oxide cyclic ester of dimethyl silicate and 120ml anisole, after the trimethylol phosphine oxide cyclic ester of dimethyl silicate is completely dissolved and dispersed, add 8.47g (0.05 mol) phosphorus trichloride, control the dropping process not to exceed 60°C, raise the temperature to 150°C after dropping, keep the temperature for 11 hours, let the hydrogen chloride be released, remove the solvent by distillation under reduced pressure, and obtain a turbid viscous liquid; then pour the crude product into Stir rapidly in 100 ml of ice water for 30 min, filter with suction, rinse with 50 ml of water, and vacuum-dry the filter cake to obtain the product tris(dimethylsily...
Embodiment 3
[0027] Example 3 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was exhausted with nitrogen, and at 20°C, 31.36g ( 0.16mol) trihydroxymethylphosphine oxide dimethylsilicate cyclic ester and 140ml xylene, after the trihydroxymethylphosphine oxide dimethylsilicate cyclic ester is completely dissolved and dispersed, add 8.47g (0.05mol ) Phosphorus trichloride, control the dropping process not to exceed 60°C, heat up to 140°C after dropping, keep the temperature for 9 hours, let the hydrogen chloride be released, and distill the solvent under reduced pressure to obtain a turbid viscous liquid; then pour the crude product into 100ml Stir rapidly in ice water for 30 min, filter with suction, rinse with 50 ml of water, and vacuum-dry the filter cake to obtain the product tris(dimethylsilylphosphonoheterocyclic methylene) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com