Muscone derivatives, preparation method and application thereof
A technology of muscone derivatives, applied in the field of preparation of muscone derivatives, can solve problems such as toxic and side effects, achieve good effects, improve brain targeting effect, and good elution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
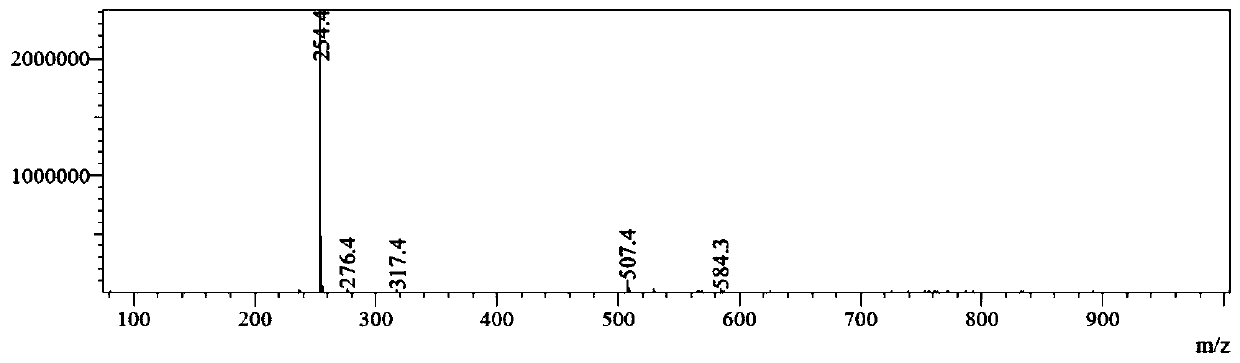
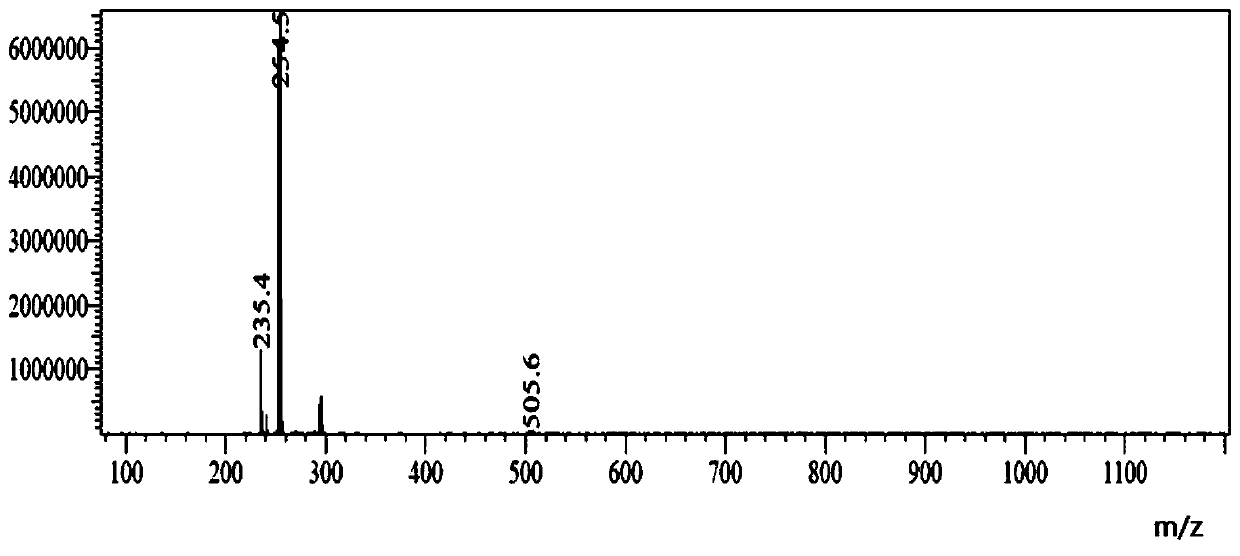
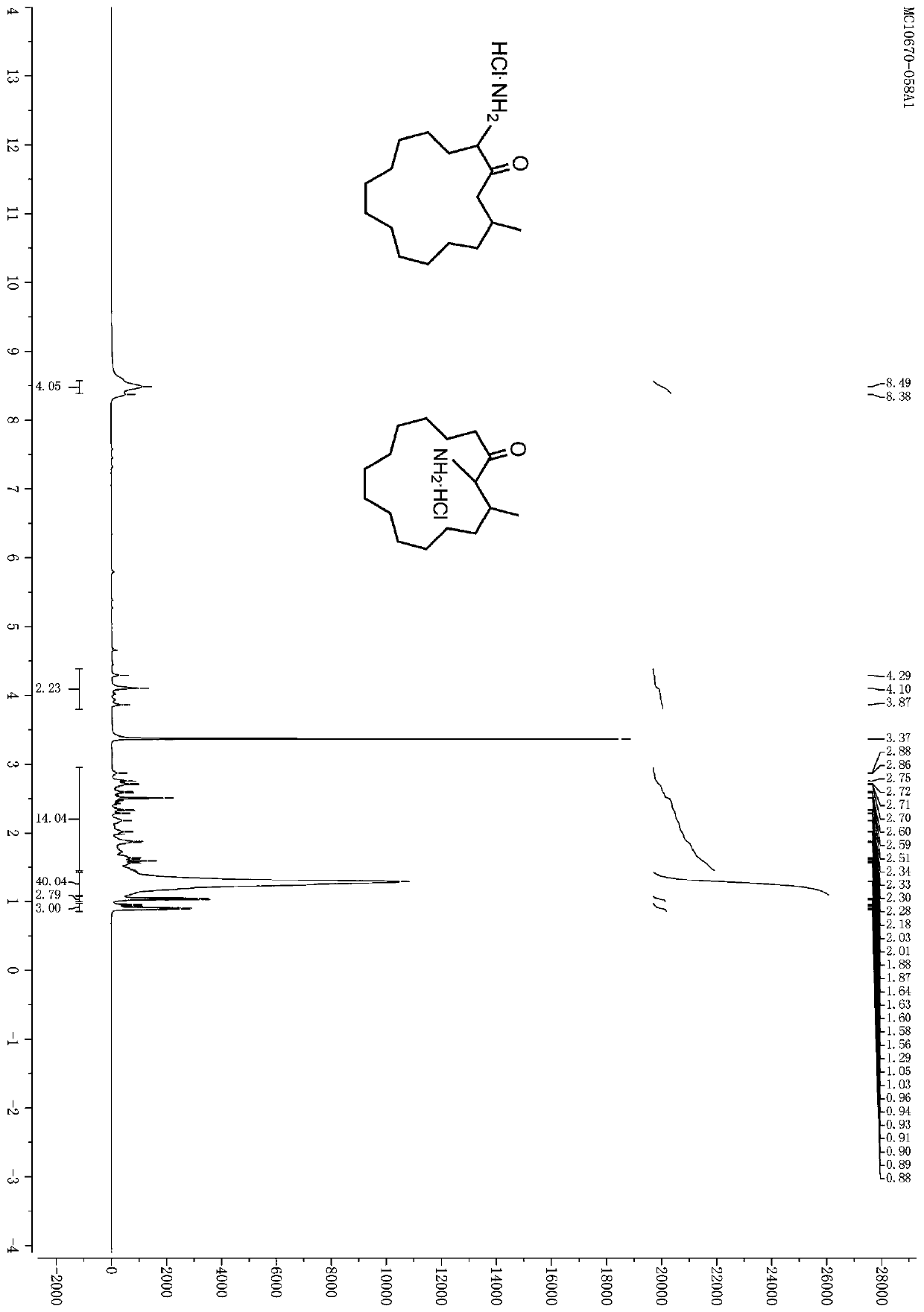
[0077] Embodiment 1: the preparation of muscone derivative
[0078] The preparation method of muscone derivative, the reaction route that described preparation method involves is:
[0079]
[0080] The concrete reaction steps of above-mentioned reaction route are:
[0081] Step 1: Synthesis of Compound C2
[0082] While stirring, add 100 g (0.63 mol) of Br to the 500 mL methanol solution containing 100 g (0.42 mol) of muscone C1 2 , and the reaction was stirred overnight at room temperature, and the resulting mixture was washed with 300 mL of saturated Na 2 S 2 o 3 The solution was diluted and extracted three times with 300 mL of ethyl acetate, the combined extracts were washed successively with 300 mL of water and 300 mL of saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4 After drying and filtering, the filtrate was concentrated and spin-dried, eluted with petroleum ether, and the residue was purified by silica gel chromatography to obtain compound ...
Embodiment 2
[0094] Embodiment 2: Muscone phospholipid compound DSPE-PEG 2000 - Preparation of muscone
[0095] The muscone phospholipid compound DSPE-PEG of the present embodiment 2000 -The preparation method of muscone, comprises the steps:
[0096] Compound C6, DSPE-PEG obtained in Example 1 2000 -The mixture of NHS and triethylamine, according to the ratio of 8mg:1mL, was dissolved in the mixed solvent of chloroform and methanol, and reacted overnight with magnetic stirring at 30°C, and the reaction mixture was washed with 50mL water and 50mL saturated NaCl aqueous solution successively, and washed with Water Na 2 SO 4 After drying and filtering, the filtrate was concentrated and spin-dried, eluted with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 10:1, and the residue was purified by chromatography to obtain the muskone phospholipid compound DSPE-PEG 2000 - muscone, its structural formula is:
[0097]
[0098] Among them, the compound C6, DSPE-PEG...
Embodiment 3
[0100] Embodiment 3: Muscone phospholipid compound DSPE-PEG 2000 - Preparation of muscone
[0101] The muscone phospholipid compound DSPE-PEG of the present embodiment 2000 -The preparation method of muscone, comprises the steps:
[0102] Compound C6, DSPE-PEG obtained in Example 1 2000 -The mixture of NHS and triethylamine, according to the ratio of 8mg:1mL, was dissolved in the mixed solvent of chloroform and methanol, and reacted overnight with magnetic stirring at 30°C, and the reaction mixture was washed with 50mL water and 50mL saturated aqueous NaCl solution successively, and washed with free Water Na 2 SO 4 After drying and filtering, the filtrate was concentrated and spin-dried, eluted with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 10:1, and the residue was purified by chromatography to obtain the muskone phospholipid compound DSPE-PEG 2000 - muscone, its structural formula is:
[0103]
[0104] Among them, the compound C6, DSP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com