Preparation method trans-tetra-substituted alkene derivative
A technology of tetrasubstituted and derivatives, which is applied in the field of preparation of trans tetrasubstituted olefin derivatives, can solve problems such as theoretical difficulties, and achieve the effects of wide substrate range, simple post-treatment, and strong reaction specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
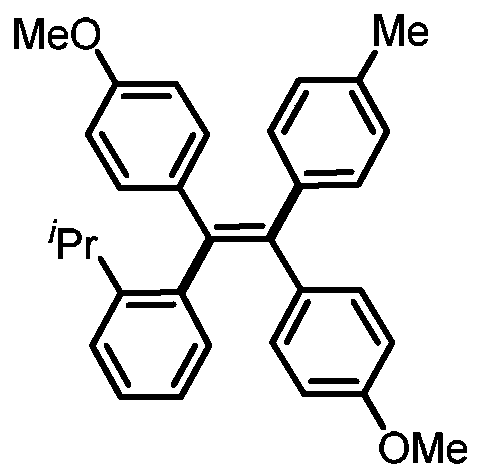
[0024] Preparation of (Z)-4,4'-(1-(o-tolyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene)
[0025]
[0026] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 2-isopropyl iodobenzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, and place it in an oil bath at 120°C. Reacted for 24h; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 34.9 mg of the target product with a yield of 78%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.17-7.12(m, 2H), 7.03-6.93(m,...
Embodiment 2
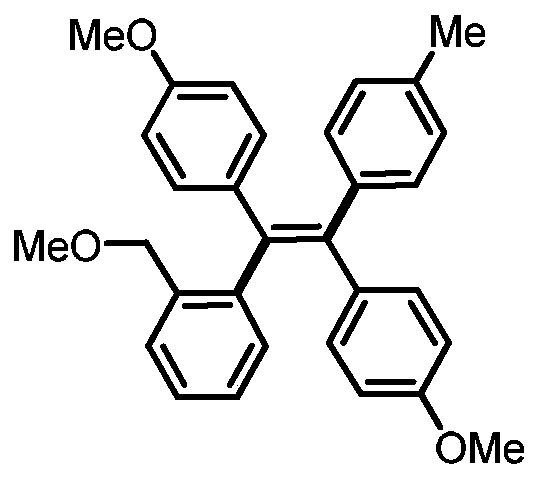
[0028] (Z)-4,4'-(1-(2-(methoxymethyl)phenyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene) preparation
[0029]
[0030] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 1-iodo-2-(methoxymethyl)benzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, and place In an oil bath at 120°C, react for 24 hours; cool to room temperature, dilute the reaction solution with ethyl acetate, wash with water three times, and wash the organic phase with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 22 mg of the target product, with a yield of 49%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.30 (dd, J = 7.8, 1.3 H...
Embodiment 3
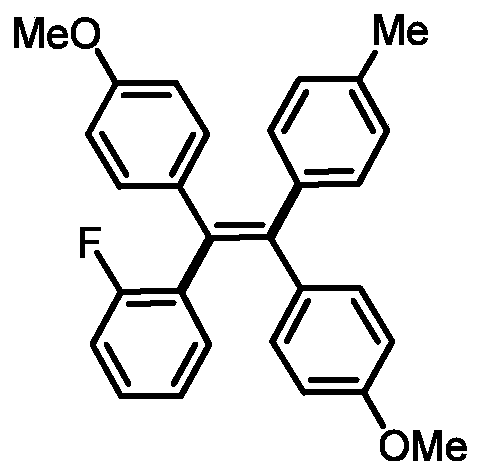
[0032] Preparation of (Z)-4,4'-(1-(2-fluorophenyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene)
[0033]
[0034] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 2-fluoroiodobenzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, place it in an oil bath at 120°C, and react for 24h ; Cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 19.9 mg of the target product, with a yield of 47%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.11-7.03(m, 3H), 6.97-6.90(m, 10H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com