Preparation method of alpha-hydroxyketone photoinitiator
A technology of photoinitiator and hydroxyketone, which is applied in the field of preparation of α-hydroxyketone photoinitiator, can solve the problems that the catalyst cannot be recycled, the reaction steps are long, and the cost is high, and the pollution is small, the reaction steps are short, and the cost is low. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
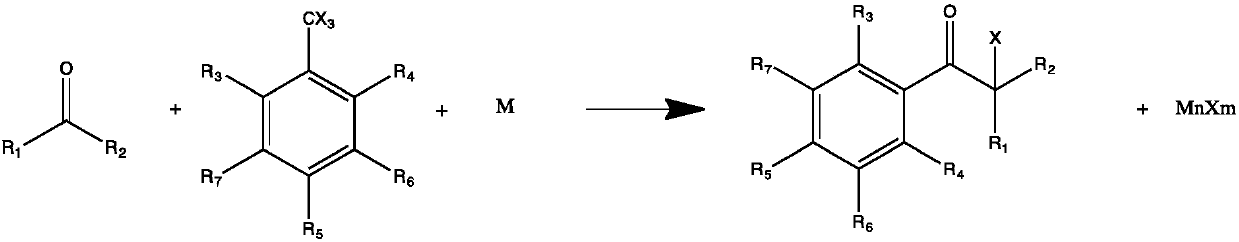
[0022] A preparation method of α-hydroxyketone photoinitiator, comprises the following steps:
[0023] Step (1): ketone compounds and trihalomethyl substituted benzene and its derivatives are used as raw materials, and react in one step under the action of a polar solvent and a metal catalyst to generate a halogenated intermediate, wherein the feed ratio of the reaction raw materials is ketones Compound: trihalomethyl-substituted benzene and its derivatives: metal catalyst = 0.9-1.5: 1: 1-10, reaction temperature: -50-40°C, general reaction formula (I):
[0024]
[0025] where R 1 , R 2 C1-C6 alkane or R 1 and R 2 Combined into cyclohexyl; R3, R4, R5, R6, R7 are hydrogen, C1-C6 alkane, C1-C6 alkoxy or C1-C6 hydroxyalkyl; X is fluorine, chlorine or bromine;
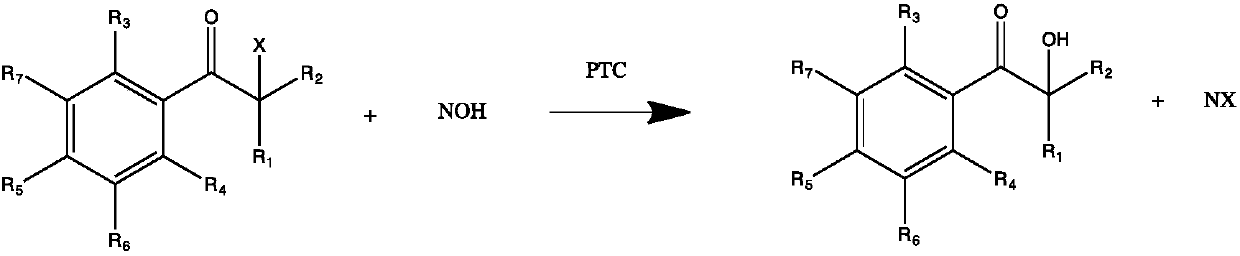
[0026] Step (2): The halogenated intermediate obtained in step (1) is hydrolyzed under the action of an aqueous alkali metal hydroxide solution and a phase transfer catalyst to obtain an α-hydroxy ketone photoinitia...
Embodiment 1
[0041] Synthesis of 1-Hydroxycyclohexylphenyl Methanone
[0042] Dissolve and suspend 12g of 80-100mesh magnesium powder in 100ml of DMF (dimethylformamide) solvent, stir and cool down to -20°C under nitrogen protection, dissolve 9.8g of cyclohexanone and 23.46g of trichloromethylbenzene in 40ml of DMF solvent Slowly add it dropwise to the system in the first step, keep the system temperature below -20-10°C and react for 2 hours, naturally warm up to room temperature, filter, wash with water, extract with petroleum ether, add 20g of 30% NaOH The aqueous solution and 0.2 g of tetrabutylammonium bromide were reacted at a temperature of 50°C for two hours, washed with water, precipitated and recrystallized to obtain 16 g of a product with a purity of 99.6% and a yield of 86%.
Embodiment 2
[0044] Synthesis of 2-Hydroxy-2-methyl-1-phenylacetone
[0045] Dissolve and suspend 12g of 80-100mesh magnesium powder in 100ml of DMF solvent, stir and cool down to -20°C under nitrogen protection, dissolve 5.8g of acetone and 23.46g of trichloromethylbenzene in 40ml of DMF solvent, and slowly add it dropwise to the first In the first step system, keep the temperature of the system below -10-0°C and react for 2 hours, naturally warm up to room temperature, filter, wash with water, extract with petroleum ether, add 35% NaOH 17g aqueous solution and tetrabutylammonium bromide 0.15g , controlling the reaction temperature to 50° C. for two hours, washing with water, precipitation and rectification to obtain 13.3 g of the product with a purity of 99.2% and a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com