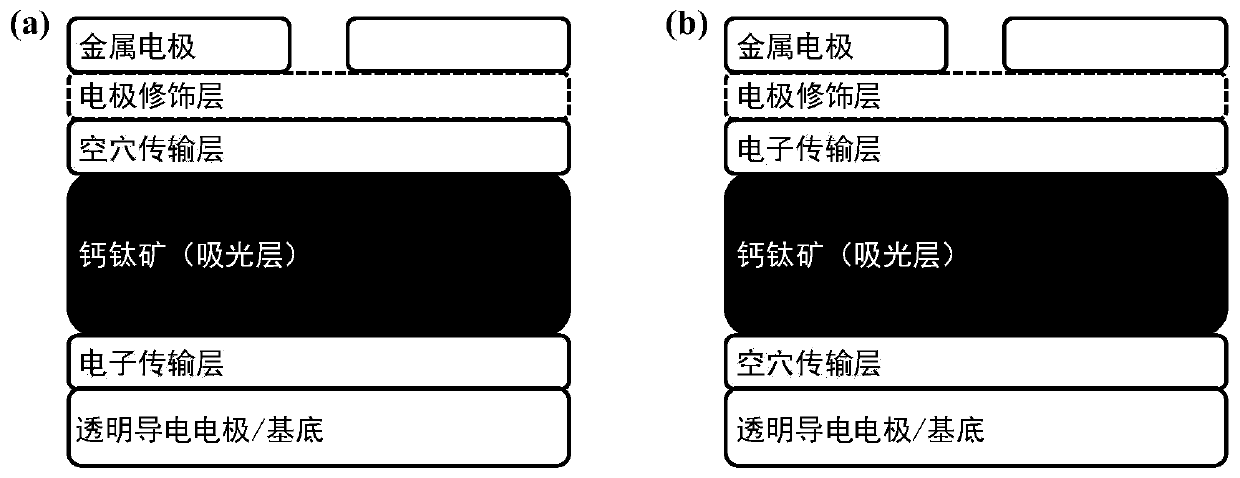
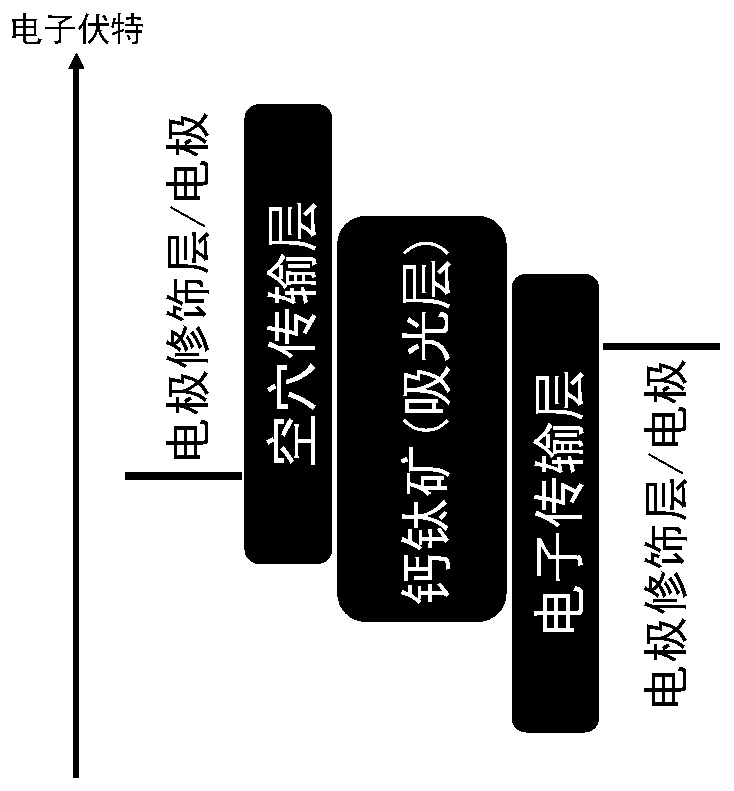
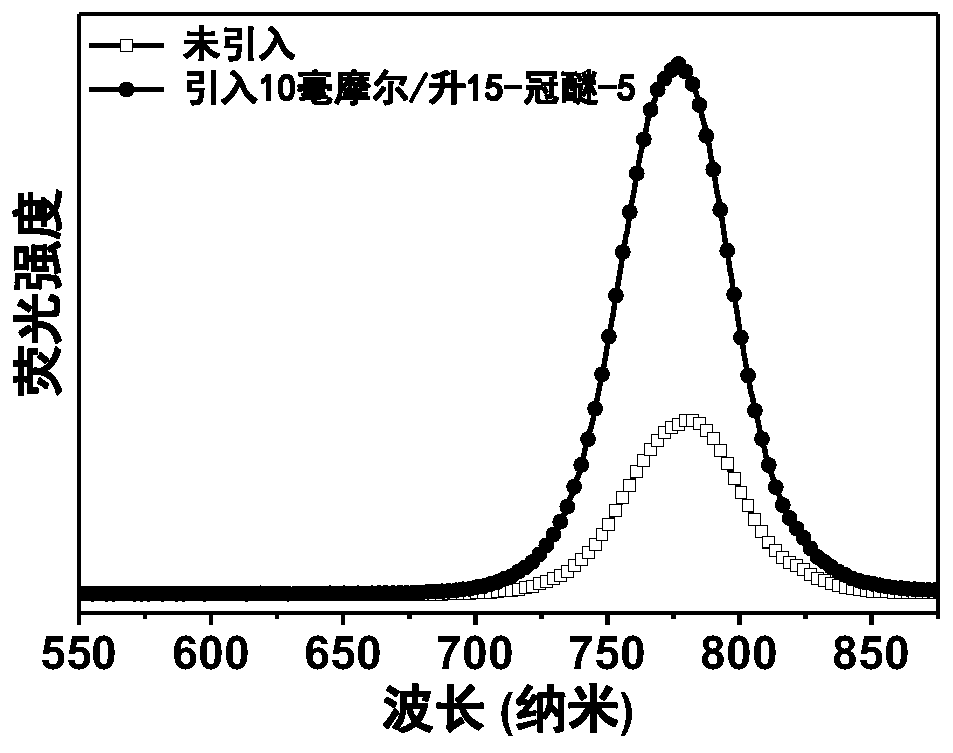
Metal halide perovskite materials, preparation method thereof as well as solar cell device and preparation method of solar cell device
A technology of metal halide and perovskite materials, which can be used in the preparation of amino compounds, organic compounds, electrical solid-state devices, etc., and can solve problems such as stability and short boards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The preparation method and specific steps of a typical laboratory-level "n-i-p" type perovskite solar cell device are described. Select mixed ionic lead halide perovskite FA 0.85 MA 0.15 Pb(I 0.85 Br 0.15 ) 3 , its precursor components formamidine iodide (FAI), lead iodide (PbI 2 ), methylamine bromide (MABr), lead bromide (PbBr 2 ) is 1.02:1.05:0.18:0.18, the solvent adopts DMSO:DMF volume ratio as a mixed solvent of 1:4, and the molar concentration of the perovskite precursor solution (calculated as lead element) is 1.2 mol / L, weighed, stirred, dissolved, filtered and set aside. The preparation method of the perovskite solution with crown ether is to weigh or measure the required amount of 15-crown ether-5 and add it to the prepared perovskite solution, stir and dissolve evenly.
[0076] Cleaning of the substrate: The FTO / glass substrate is ultrasonically cleaned for about 20 minutes with detergent / water, deionized water, acetone, and isopropanol in sequence, a...
Embodiment 2
[0090] The preparation method and specific steps of a typical laboratory-level "n-i-p" type perovskite solar cell device are described. We prefer mixed ionic lead halide perovskite FA 0.85 MA 0.15 Pb(I 0.85 Br 0.15 ) 3 , its precursor components formamidine iodide (FAI), lead iodide (PbI 2 ), methylamine bromide (MABr), lead bromide (PbBr 2 ) is 1.02:1.05:0.18:0.18, the solvent adopts DMSO:DMF volume ratio as a mixed solvent of 1:4, and the molar concentration of the perovskite precursor solution (calculated as lead element) is 1.2 mol / L, weighed, stirred, dissolved, filtered and set aside. The preparation method of the perovskite solution with crown ether is to weigh or measure an appropriate amount of dibenzo-21-crown ether-7 and add it to the prepared perovskite solution, stir and dissolve evenly.
[0091] Cleaning of the substrate: The FTO / glass substrate is ultrasonically cleaned for about 20 minutes with detergent / water, deionized water, acetone, and isopropanol i...
Embodiment 3
[0101] The preparation method and specific steps of a typical laboratory-level "n-i-p" type inorganic perovskite solar cell device are described. Select the inorganic lead halide perovskite CsPbI 2 Br, its precursor components cesium iodide (CsI), lead iodide (PbI 2 ), lead bromide (PbBr 2 The molar ratio of substances between ) is 1:0.5:0.5, the solvent adopts DMF:DMSO volume ratio as a mixed solvent of 1:4, and the molar concentration of the inorganic perovskite precursor solution (calculated by lead element) is 0.7 moles / L, weighed, stirred, dissolved, filtered, and set aside. The method for preparing the inorganic perovskite solution with crown ether is to weigh or measure an appropriate amount of 18-crown ether-6 and add it to the prepared inorganic perovskite solution, stir and dissolve evenly.
[0102] Cleaning of the substrate: The FTO / glass substrate is ultrasonically cleaned for about 20 minutes with detergent / water, deionized water, acetone, and isopropanol in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com