Perfluoropolyether modified dentritic silane derivative, as well as preparation method and application thereof
A technology of silane derivatives and perfluoropolyether, which is applied in the direction of polyether coatings, biocide-containing paints, coatings, etc., can solve the problems of initial contact angle and wear resistance that cannot meet the needs of high-end application products, and achieve Excellent wear resistance, simple process, excellent anti-fingerprint effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] And, the embodiment of the present invention provides a method for preparing perfluoropolyether-modified dendritic silane derivatives, comprising the following steps:
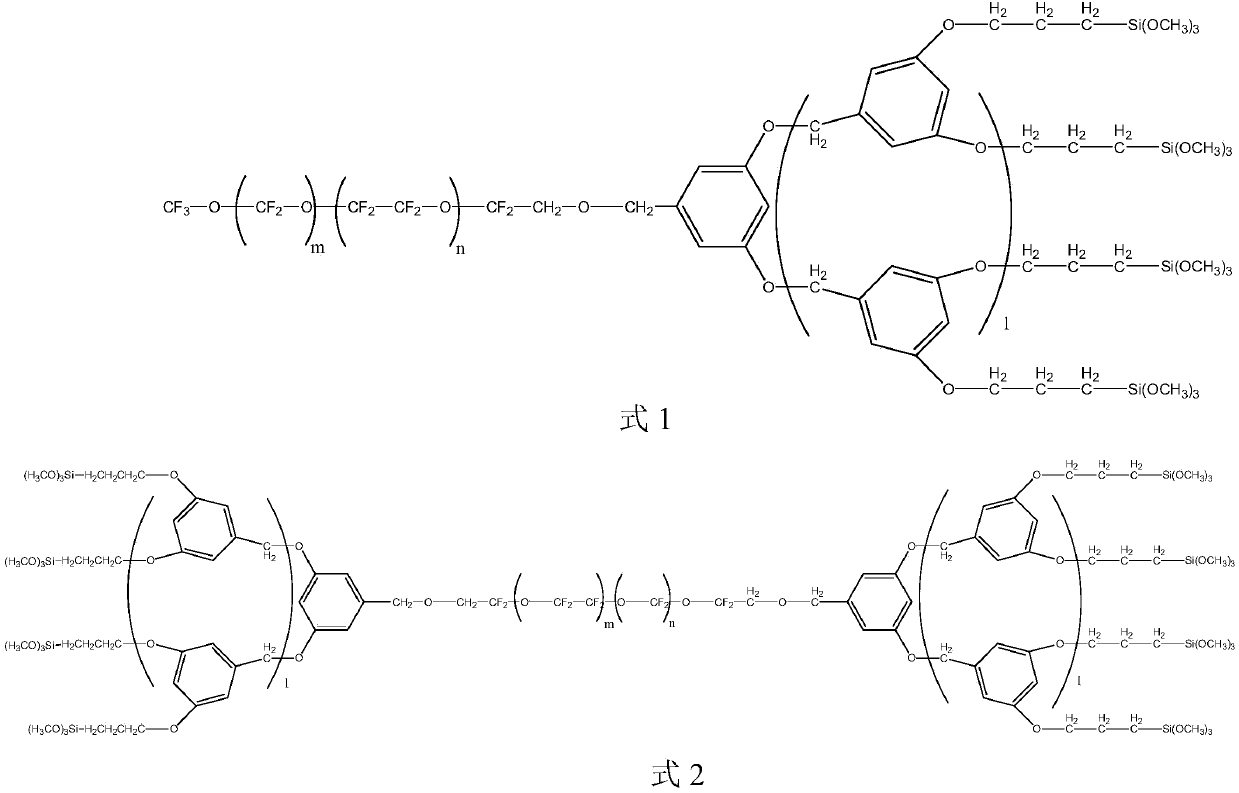
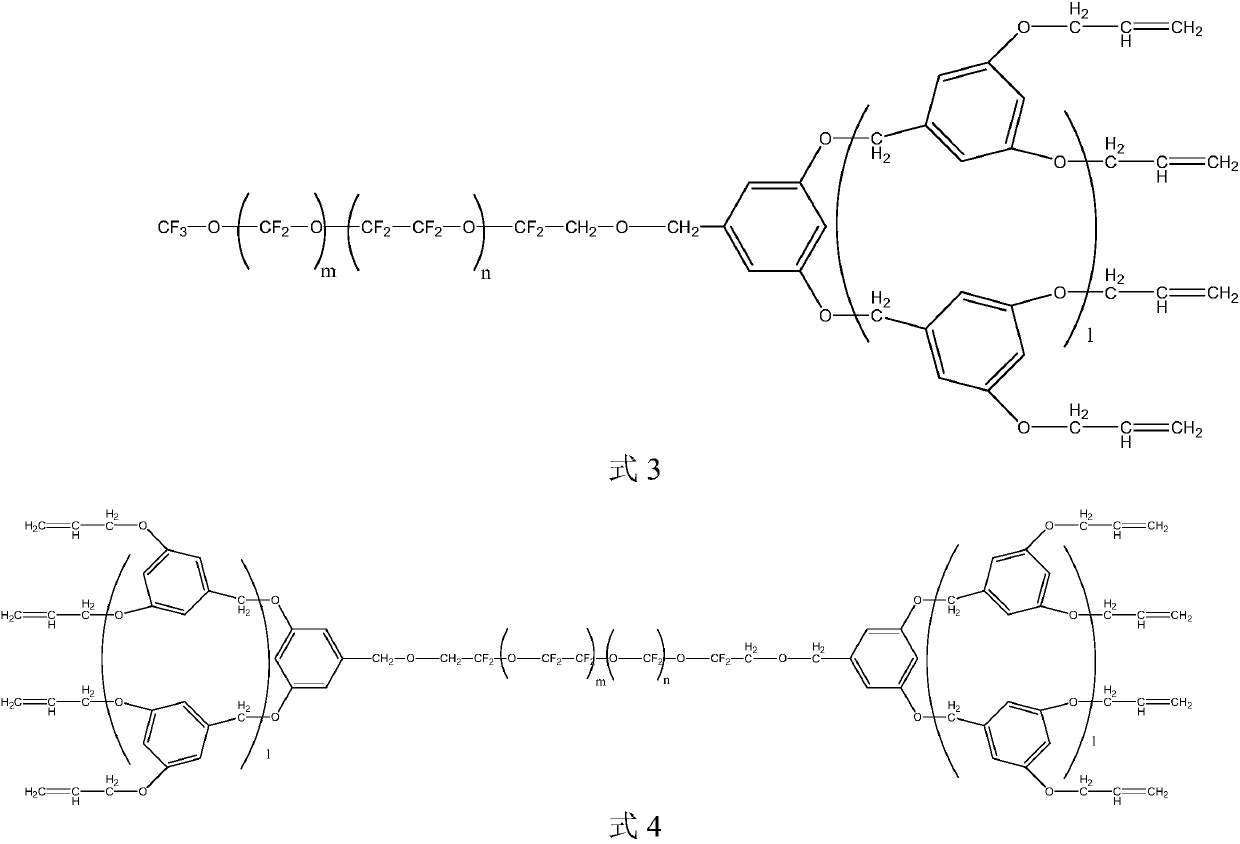
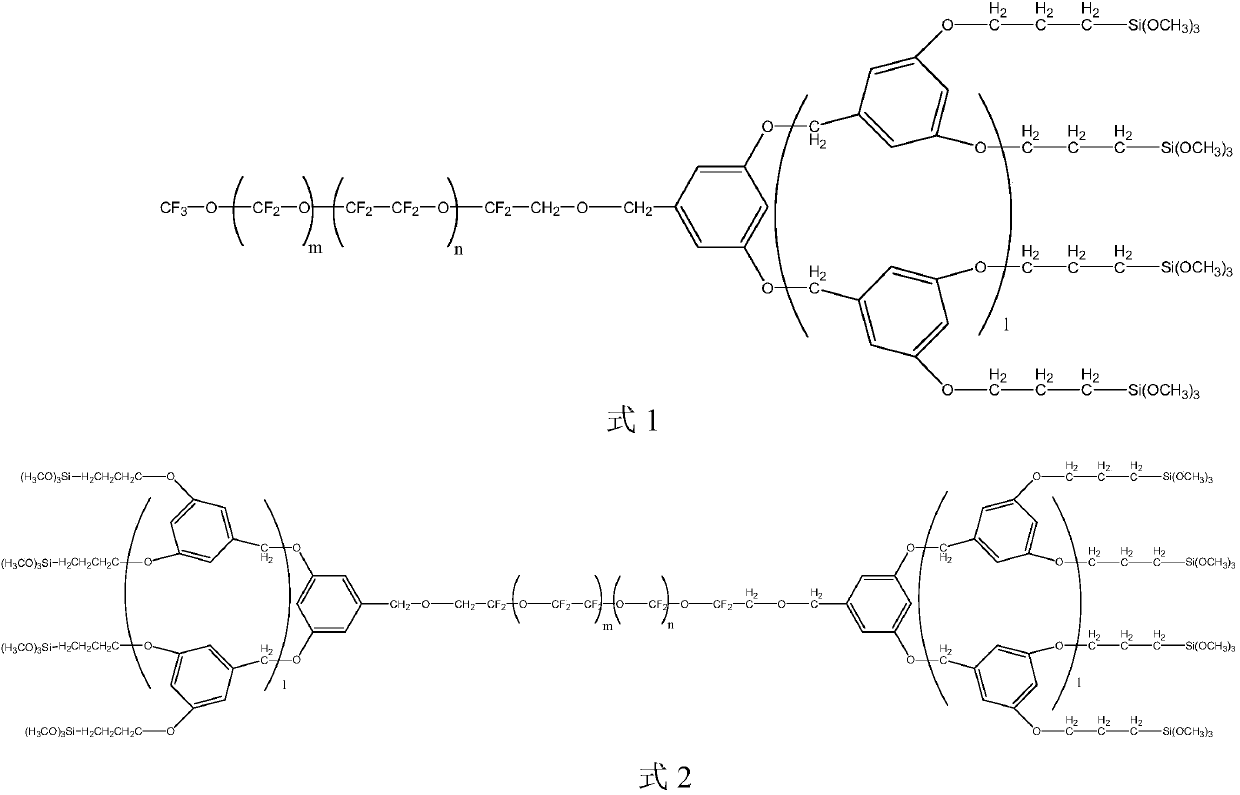
[0028] S01. Prepare the perfluoropolyether-modified dendritic polymer intermediates shown in the following formula 3 and / or formula 4 respectively, wherein m and n are respectively selected from the integers of 1 to 40, and m+n=20~ 60, m / n=0.3~3; l is 0 or 1;
[0029]
[0030]
[0031] S02. Under nitrogen or oxygen protection, the dendrimer intermediate of the perfluoropolyether modification shown in formula 3 and / or formula 4 is mixed with 1,3-bis-(trifluorotoluene), trialkoxy Hydrogen silane mixed, add catalyst, react at 40-116 °C for 4-24h, prepare the compound shown in formula 1 and / or formula 2, wherein, the alkoxy group in the trialkoxy hydrosilane is selected from An alkoxy group having 1 to 5 carbon atoms.
[0032] The preparation method of the perfluoropolyether-modified dendritic silane...
Embodiment 5
[0063] Example 5,5'-((5-(bromomethyl)-1,3-phenylene)bis(oxyl))bis(methylene))bis(1,3-bis(allyl) The reaction conditions of oxy)benzene) and perfluoropolyether alcohol are: react at 40-110°C for 4-12h. If the reaction temperature is too low and the time is too short, the raw materials cannot be converted well, and if the reaction temperature is too high and the time is too long, more side reactions will occur, which will affect the product yield.
[0064] After the reaction, the reaction system was washed with water until the pH of the lower layer solution was 7, water-soluble impurities were removed, the lower layer solution was collected, and the rotary evaporation was performed to remove low boilers (using the boiling point of the product as a standard). Wash with an organic solvent to remove unreacted raw materials, collect the viscous material in the lower layer, and concentrate under reduced pressure to obtain the compound represented by formula 3 and / or formula 4 when l=...
Embodiment 1
[0075] A dendritic silane derivative modified by perfluoropolyether, the structural formula of the dendritic polymer modified by perfluoropolyether is shown in the following formula (named S-1):
[0076]
[0077] Wherein, m and n are respectively selected from integers of 1-40, and m+n=20-60, m / n=0.3-3; l is 0.
[0078] The preparation method of above-mentioned compound comprises the following steps:
[0079] S11. Synthesis of Perfluoropolyether Modified Dendrimer Intermediates
[0080] Add 202g of 5-bromomethyl-1,3-benzenediol, 250g of allyl bromide, 1000g of THF (tetrahydrofuran), and 20g of a phase transfer catalyst in a four-necked flask equipped with a thermometer, a stirring device, a condensing device, and a vacuum distillation device TBAB (tetrabutylammonium bromide), 250g of 40% sodium hydroxide aqueous solution, mixed and stirred, heated to 66°C for 4 hours, distilled off the solvent under reduced pressure, added 500g of water, stirred and washed for 30 minutes, al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com