Block copolymers of lactones and poly(propylene fumarate)
A kind of technology of propylene glycol fumarate and block copolymer, applied in the field of preparation and functionalization of the polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
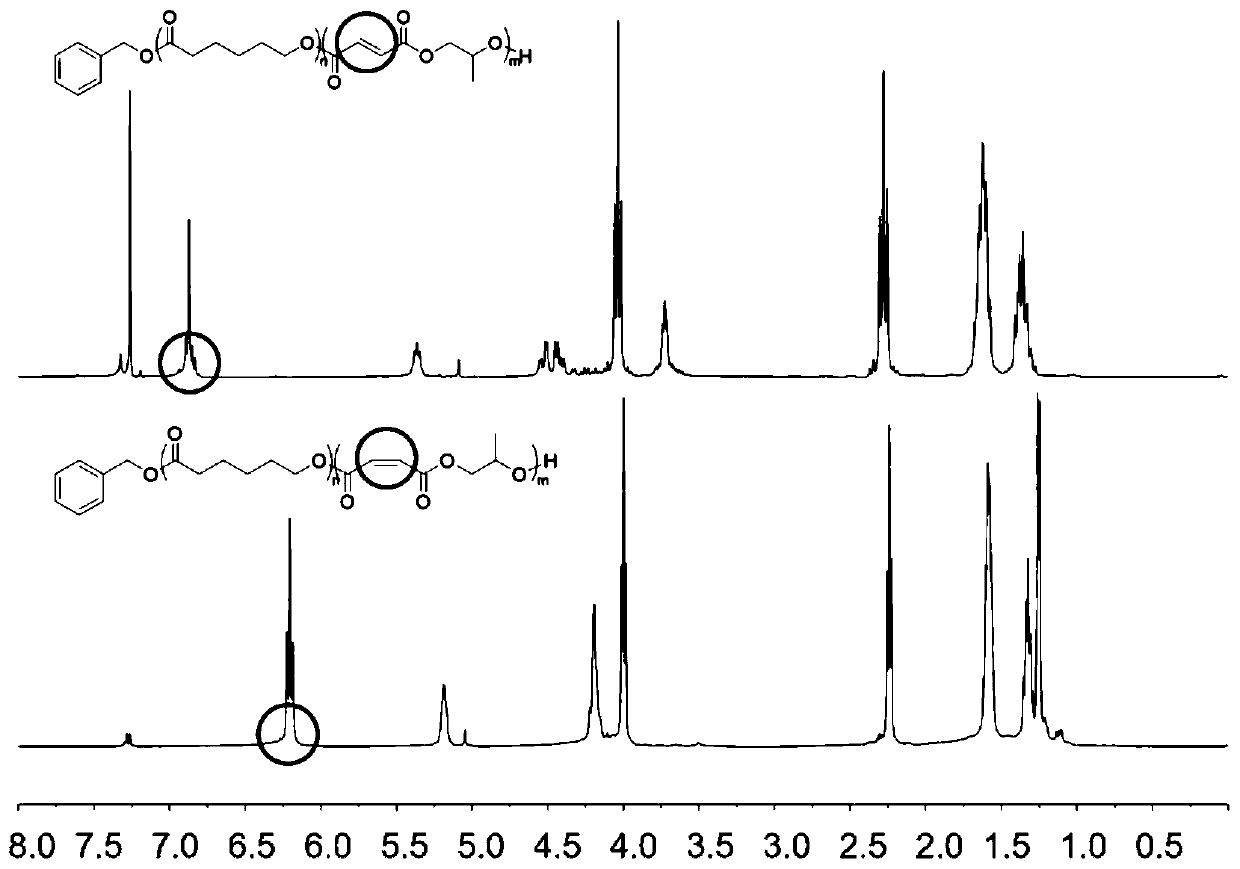
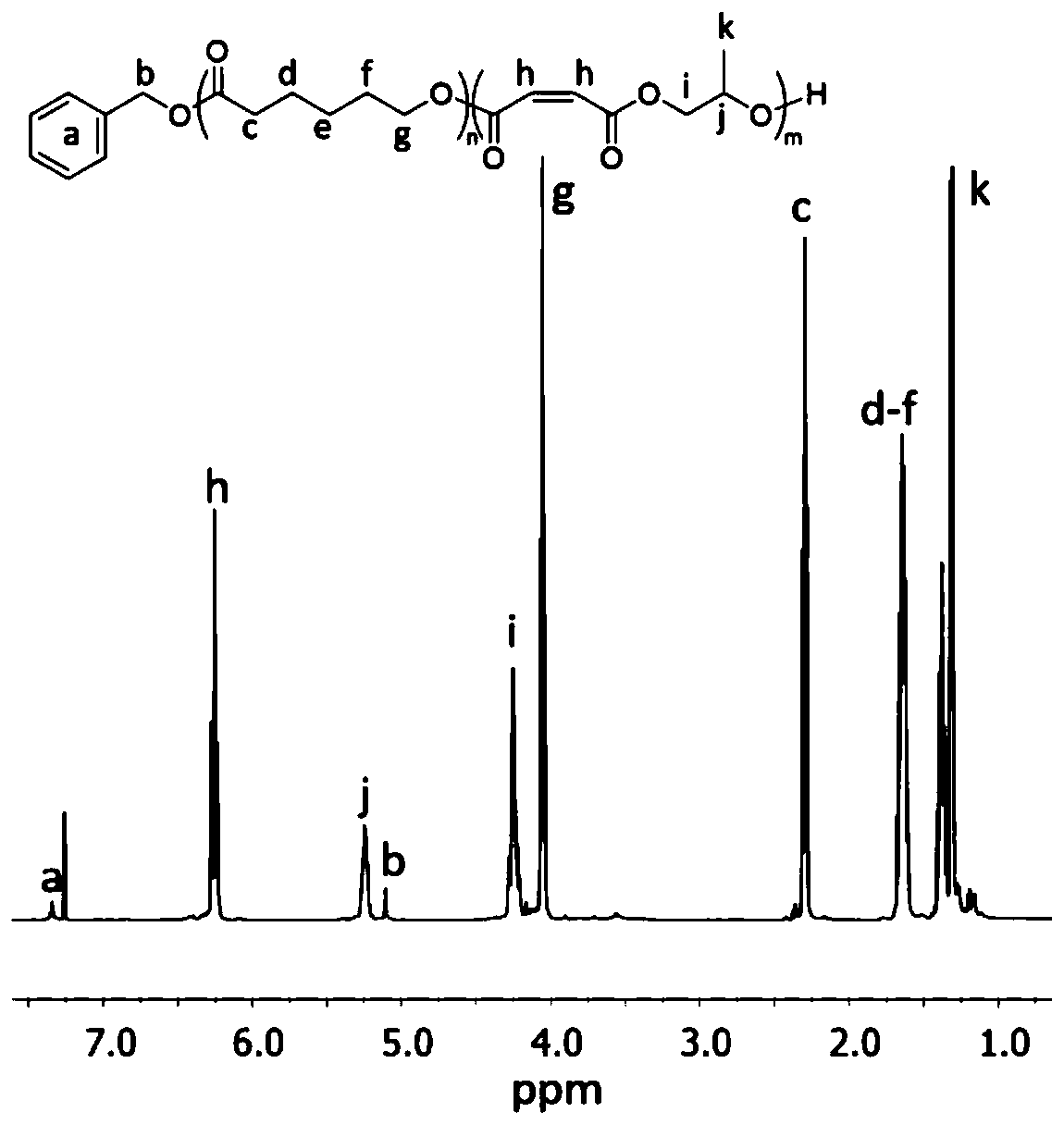
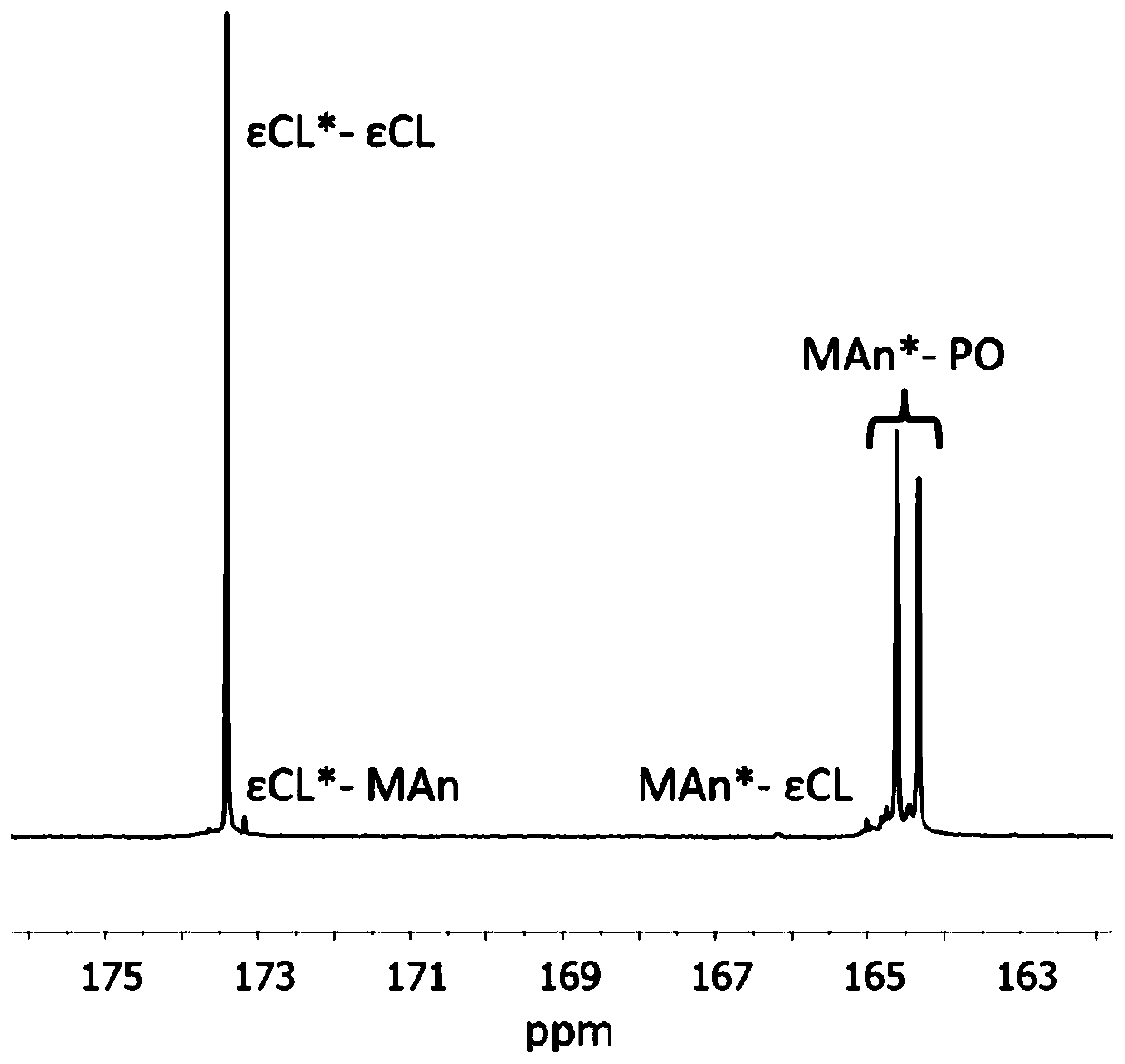
[0182] The ROCOP synthesis of δVL and εCL was also studied, followed by the ROCOP of MAn and PO. Use Mg again (BHT) 2 (THF) 2 As a catalyst and BnOH as an initiator, δVL and εCL were copolymerized in toluene at a concentration of 2M at 80°C for 24 hours. 1 The H NMR spectrum showed that in addition to MAn and PO, δVL and εCL were equally incorporated into the polymer chain. by 13 C NMR spectroscopy analysis of the carbonyl binary resonance peak shows a sequence-like block, where δVL and εCL are randomly incorporated into the first block, and MAn and PO are alternately incorporated into the second block. DOSY NMR spectroscopy confirmed the existence of a single diffusive polymer species.
[0183] Thermal analysis using differential scanning calorimetry (DSC) reveals a wide range of properties. (See Figure 21-28 ). As expected, the polymer containing the aliphatic lactone block has a melting temperature higher than room temperature and a crystallization temperature higher than 0...
Embodiment 1
[0197] Synthesis of ε-heptanolide and γ-methyl-ε-caprolactone
[0198] The single-neck round bottom flask containing 250 mL of dichloromethane was cooled in an ice bath, and then 223 mmol of 2-methylcyclohexanone or 4-methylcyclohexanone and 275 mmol of m-chloroperoxybenzoic acid were added. After refluxing for 3 days, the reaction mixture was cooled in an ice bath, filtered with Celite, and used 10% Na 2 S 2 O 3 Solution, saturated Na 2 CO 3 Solution and brine wash. Then use MgSO 4 The organic layer was dried and filtered, and then the solvent was removed by rotary evaporation. The two products were dried overnight on calcium hydride and distilled under vacuum before use.
[0199] ε-Heptanolide :
[0200] by 1 H NMR (300MHz, 303K, CDCl 3 ) Confirm the presence of ε-heptanolide: δ = 4.44 (m, CH 2 (CH 3 )O), 2.64(m,C(=O)CH 2 ), 1.73(m,CH 2 CH(CH 3 )), 1.42(m,CH 2 CH 2 CH(CH 3 )).
[0201] γ-methyl-ε-caprolactone :
[0202] by 1 H NMR (300MHz, 303K, CDCl 3 ) Confirm the presence of γ...
Embodiment 2
[0204] Synthesis of θ-propargyl-ε-nonanolactone
[0205] 30.0mL cyclohexanone (28.4g, 289mmol), 28.5mL pyrrolidine (24.7g, 347mmol) and 55.0mg p-toluenesulfonic acid monohydrate (0.289mmol) were dissolved in 60mL toluene in a round bottom flask. The bottom flask is equipped with a Dean-Stark device and a reflux condenser. The solution was stirred at 150°C for 16 hours. The resulting solution was washed and cooled to room temperature, washed with water and brine, and then washed with MgSO 4 Dry and remove the solvent under reduced pressure. The product was purified by vacuum fractional distillation (31.4 g, boiling point 107-114°C) to obtain a pale yellow oil. Then it was dissolved in dry MeCN in a double-necked round bottom flask equipped with a reflux condenser, and then 26.8 mL of propargyl bromide (80% toluene solution, 37.0 g, 249 mmol) was added dropwise to the solution. in. The reaction was stirred overnight under reflux, then cooled to room temperature, and then the so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com