Coumarin oxime ester photoinitiator
A photoinitiator, coumarin oxime technology, applied in the field of a class of photoinitiator containing coumarin unit and oxime ester unit and its preparation, can solve the problems of poor activity, slow printing speed, small absorption cross section, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
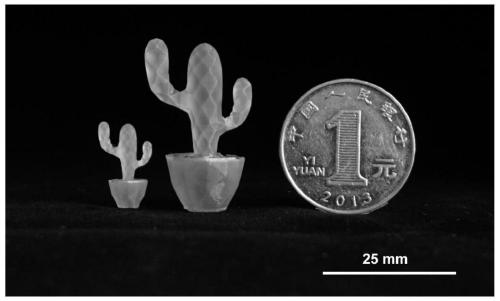
Image
Examples
Embodiment 1
[0069]
[0070] The preparation of coumarin oxime ester photoinitiator H1, concrete preparation steps are as follows:
[0071] (1) Synthesis of A1
[0072] In a 50ml single-necked round bottom flask, add 3.37g (30.9mmol) 3-aminophenol, 1.25g yttrium nitrate hexahydrate (Y(NO 3 ) 3 ·6H 2O) (3.3mmol) and 4.82g (37.1mmol) ethyl acetoacetate, reacted at 90 ° C for 2 hours, then cooled to room temperature, dissolved the reactant with 50ml ethanol, then poured it into a large amount of water, and filtered it to obtain yellow The solid was dried in a vacuum oven at 40° C. to obtain 2.58 g of the final product, with a yield of 50%.
[0073]
[0074] A1 NMR characterization: 1 H NMR (400MHz, Chloroform-d) δ7.39(dd, J=2.3,1.2Hz,1H),6.61(d,J=2.3Hz,1H),6.59(d,J=1.2Hz,1H),6.05 (q, J=1.2Hz, 1H), 4.17(s, 2H), 2.38(d, J=1.2Hz, 3H).
[0075] (2) Synthesis of B1
[0076] Disperse 3.25g (18.6mmol) of A1 in 90ml of water, add 7ml of concentrated sulfuric acid dropwise, and then add 1...
Embodiment 2
[0096]
[0097] The synthesis of H2, the specific steps are as follows:
[0098] (1) Synthesis of C1
[0099] In a 100ml reaction flask, 1.5g (6.8mmol) of 3-iodophenol and 2g (21.1mmol) of anhydrous magnesium chloride were dissolved in 50ml of anhydrous acetonitrile and 6ml (43.2mmol) of triethylamine solution, and then 2g (66.7 mmol) paraformaldehyde, reacted at 85°C for 24 hours; the reaction was cooled to room temperature, then neutralized with 1N HCl solution, extracted three times with dichloromethane, anhydrous Na 2 SO 4 Drying and rotary evaporation gave a crude product, which was finally separated and purified by silica gel column chromatography to obtain 0.69 g of the product, with a yield of 40%.
[0100]
[0101] C1 NMR characterization: 1 H NMR (400MHz, Chloroform-d) δ11.05(s, 1H), 9.87(s, 1H), 7.46(s, 1H), 7.42(dd, J=8.1, 1.5Hz, 1H), 7.26(d, J = 8.1 Hz, 1H).
[0102] (2) Preparation of D1
[0103] In a 50ml single-necked round bottom flask, dissolve 0....
Embodiment 3
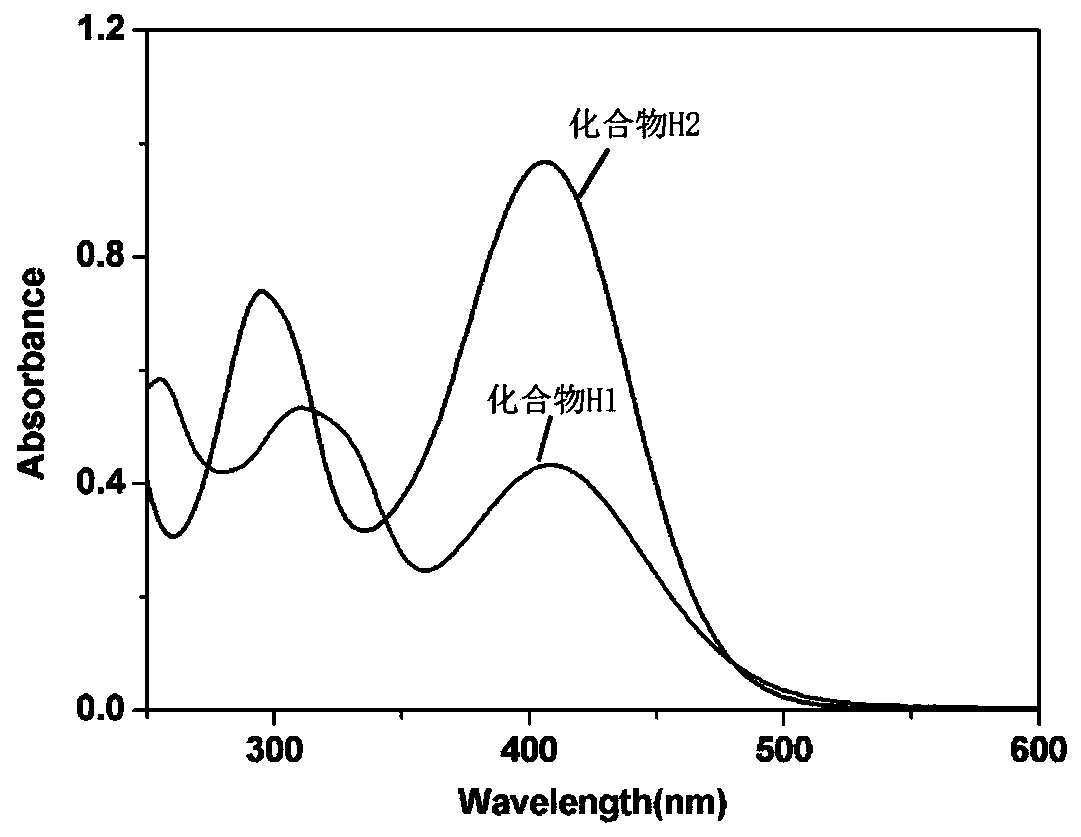
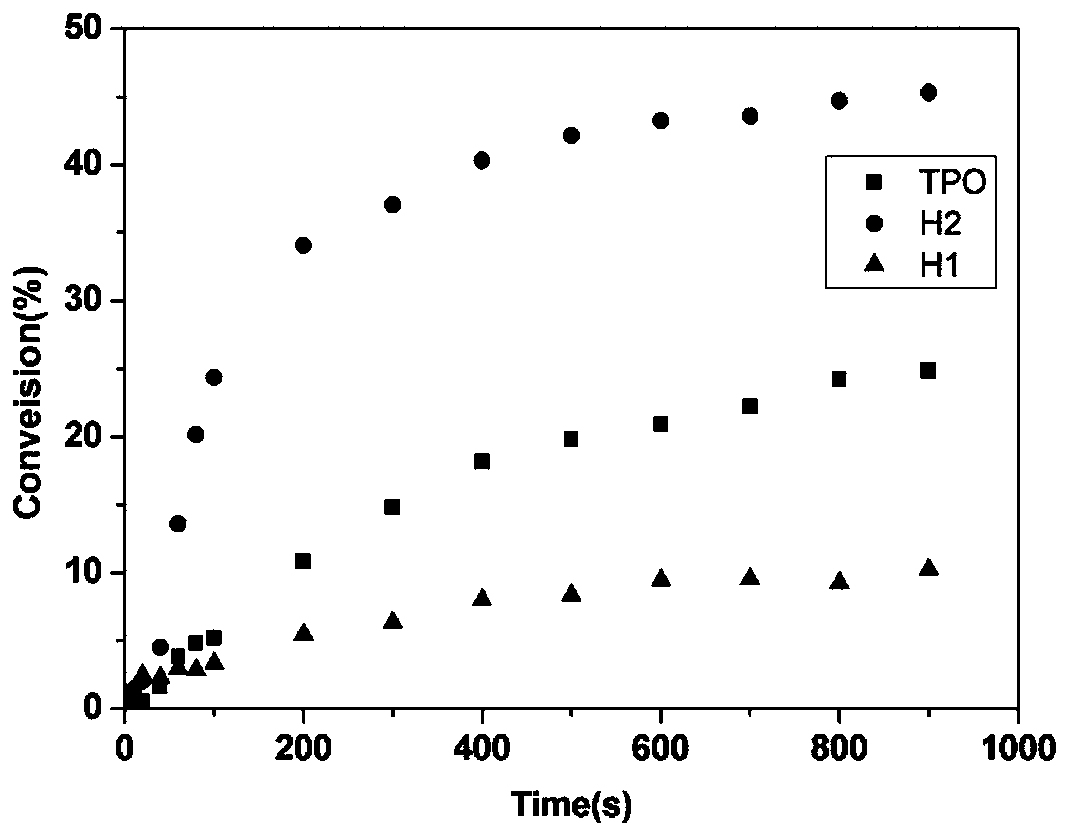
[0119] Compound H1 and compound H2 prepared in Example 1 and Example 2 are respectively subjected to performance measurement, and its absorption band is 360-450nm as measured by an ultraviolet-visible spectrophotometer, and its ultraviolet-visible absorption spectrum is shown in figure 1 , it can be found that its maximum absorption wavelength is around 405nm, which matches the wavelength of the longest used 405nm LED lamp.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com