Expression box capable of efficiently achieving secretory expression of human FGF21 protein and application of expression box
A technology for expression cassettes and proteins, applied in the biological field, can solve the problems of low secretion efficiency, slow growth, hidden dangers in production safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] 3. Preparation and transformation of DNA:
[0039] DNA was isolated from E. coli and B. subtilis or from agarose gels using DNA preparation kits from Tiangen or Omega according to the manufacturer's instructions. Standard molecular techniques were used in all examples. Escherichia coli was transformed using plasmid DNA as described by Chung C.T. et al., Proc. According to a modified "Paris method" (Harwood C.R. Molecular Biological Methods for Bacillus, 1990, John Wiley & Sons Ltd., England), plasmid DNA or DNA fragments were used to transform Bacillus subtilis.
[0040] 4. Bacillus subtilis gene traceless knockout:
[0041] Gene knockout was performed according to the improved "AraR-based Bacillus subtilis gene knockout method" (Liu S, Endo K, et al. Microbiology. 2008; 154(Pt 9): 2562-2570.). First, using the principle of homologous recombination, the spectinomycin resistance gene fragment "Para-spc" carrying the homology arm and regulated by the arabinose promoter...
Embodiment 1
[0056] Example 1. Construction of rhFGF21 expression cassette containing cistron cistron Optimizing rhFGF21 translation efficiency to promote protein expression level
[0057] 1.1 Design of cistron-containing rhFGF21 expression cassette
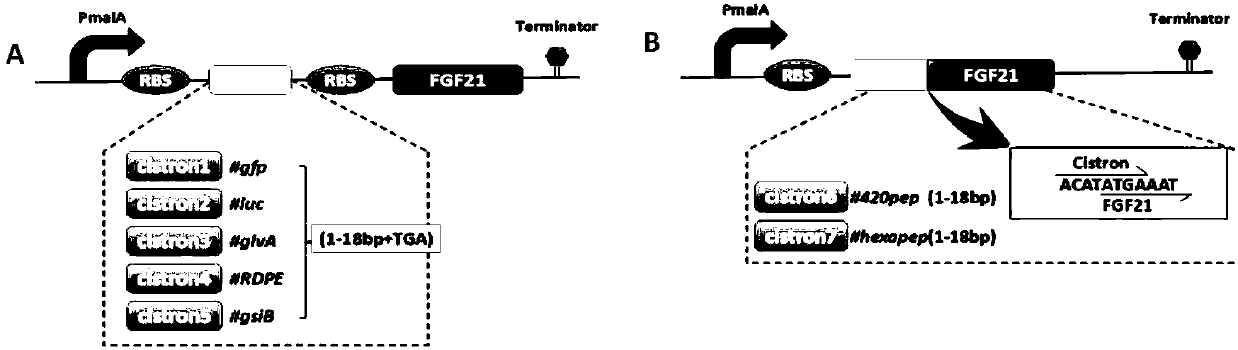
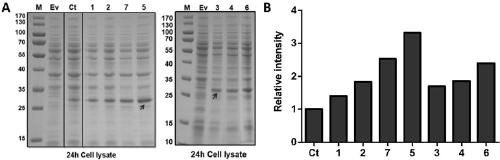
[0058] In order to increase the expression level of rhFGF21, the present invention inserts the cistron cistron into the rhFGF21 expression vector to form a rhFGF21 expression cassette containing the cistron cistron. The purpose of inserting cistron is to change the secondary structure of mRNA. The RBS is exposed from the original stem-loop structure, which facilitates the recruitment of more ribosomes, enhances the combination of ribosomes and RBS, and improves the translation efficiency of proteins. figure 1 It is a schematic diagram of the structure of the rhFGF21 expression cassette containing the cistron cistron. figure 1 The structure of A is: promoter-RBS-cistron(1-5)-RBS-rhFGF21-terminator, cistron1-cistron5 are the first 18bp of gfp,...
Embodiment 2
[0062] Example 2. Helping rhFGF21 to fold correctly by optimizing disulfide bonds to obtain high-yield soluble expression
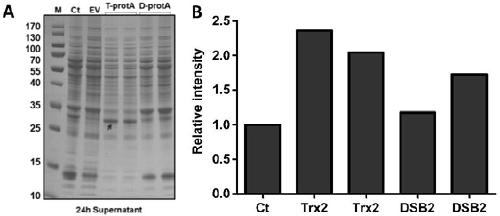
[0063] The rhFGF21 protein contains a disulfide bond inside. Since the type and expression level of disulfide bond-related foldases in Bacillus subtilis are relatively low, there are risks such as incorrect formation or formation of misfolded disulfide bonds in the FGF21 protein. Aiming at this situation, the present invention uses the pDL vector (derived from BGSC) as the backbone to clone and construct the integrated vector pDF-d carrying the oxidoreductase DsbA (the construction method is the same as in Example 1) and construct the weakened thioredoxin The carrier ptrx1 of the protein TrxA, that is, the promoter of the gene TrxA is replaced by the promoter P with weaker expression strength spac (construction method is the same as embodiment 1). The vectors pDF-d and ptrx1 were respectively transformed into Bacillus subtilis 1A751, and the mutant strai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com