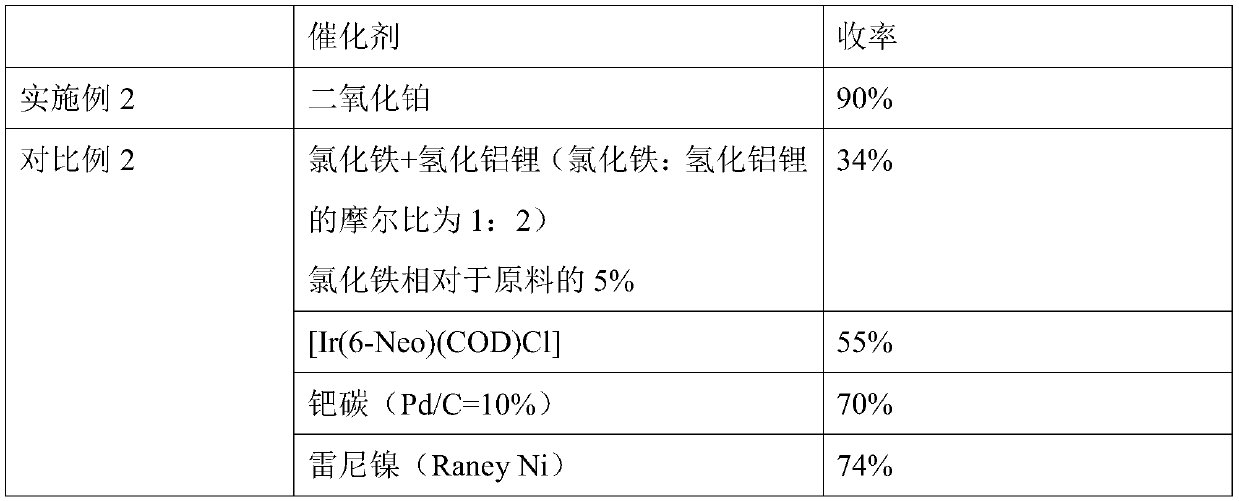
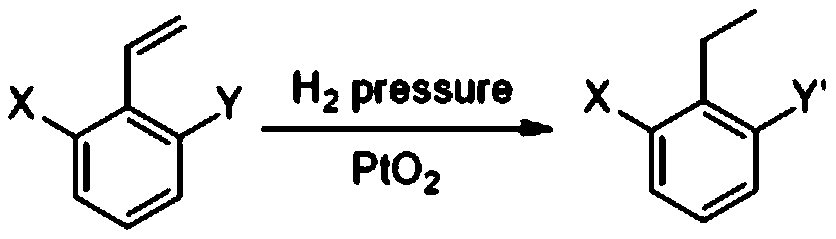A method for hydrogenation reduction of alkenyl aromatic halogenated derivatives
A technology of aromatic halogenation and derivatives, which is applied in the field of organic synthesis, can solve the problems of cumbersome cost, difficult thoroughness, cumbersome process, etc., and achieve the effect of improving reaction selectivity and yield, reducing post-processing cost, and reducing catalytic cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1. A method for hydrogenation reduction of alkenyl aromatic halogenated derivatives, using 2-chloro-6-nitrostyrene as raw material, the following steps are carried out in sequence:
[0045] 1) At room temperature, add 10.00g (54.5mmol) of 2-chloro-6-nitrostyrene and 50mL of anhydrous methanol into a 100ml pressure bottle, add a magnetic rotor to keep stirring, and add 0.50g of platinum dioxide after the solution is clear (that is, 0.05 mass times for the raw material), keep fully stirred evenly;
[0046]2) At room temperature, pressurize the system obtained in step 1) with hydrogen: first replace the air in the pressure bottle with low-pressure hydrogen (0.02mpa), and then maintain the hydrogen pressure in the system (that is, the hydrogen pressure in the pressure bottle) to 0.05mpa, keep stirring for 6h, and TLC detects that the reaction is complete.
[0047] 3), pass the reaction solution obtained in step 2) directly through a layer of 1 cm thick diatomace...
Embodiment 2
[0049] Embodiment 2, a method for hydrogenation reduction of alkenyl aromatic halogenated derivatives, using 2-bromo-6-aminostyrene as raw material, the following steps are carried out in sequence:
[0050] 1) At room temperature, add 8.0g (40.4mmol) of 2-bromo-6-aminostyrene and 40mL of absolute ethanol into a 100ml pressure bottle, add a magnetic rotor to keep stirring, and add 0.40g of platinum dioxide (raw material 0.05 mass times), keep fully stirring evenly;
[0051] 2) At room temperature, pressurize the system obtained in step 1) with hydrogen, first replace the air in the pressure bottle with low-pressure hydrogen (0.02mpa), then maintain the hydrogen pressure of the system at 0.05mpa, keep stirring for 3h, and detect by TLC The reaction is complete.
[0052] 3), the reaction solution obtained in step 2) is directly passed through a layer of 1 cm thick diatomaceous earth (so as to realize filtration), and the obtained filtrate is concentrated to dryness (pressure of ...
Embodiment 3
[0054] Embodiment 3. A method for hydrogenation reduction of alkenyl aromatic halogenated derivatives, using 2,6-diiodostyrene as a raw material, and performing the following steps in sequence:
[0055] 1) At room temperature, add 5.00g (13.9mmol) of 2,6-diiodostyrene and 25mL of anhydrous methanol into a 100ml pressure bottle, add a magnetic rotor to keep stirring, and add 0.25g of platinum dioxide (raw material 0.05 mass times), keep fully stirring evenly;
[0056] 2) At room temperature, pressurize the system obtained in step 1) with hydrogen, first replace the air in the pressure bottle with low-pressure hydrogen (0.02mpa), then maintain the hydrogen pressure of the system at 0.05mpa, keep stirring for 4h, and detect by TLC The reaction is complete.
[0057] 3), the reaction solution obtained in step 2) was directly passed through a layer of 1 cm thick diatomaceous earth (so as to achieve filtration), and the obtained filtrate was concentrated to dryness to obtain 6.2 g o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com