Process for recycling and regenerating lithium cobalt oxide in waste lithium cobalt oxide battery
A technology of lithium cobaltate and lithium cobaltate, which is applied in battery electrodes, secondary batteries, battery recycling, etc., can solve the problems of low purity of precipitated recovered products, large impact of inorganic acids on the environment, and low utilization rate of elements. Obvious social value, low equipment requirements, and simple regeneration process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
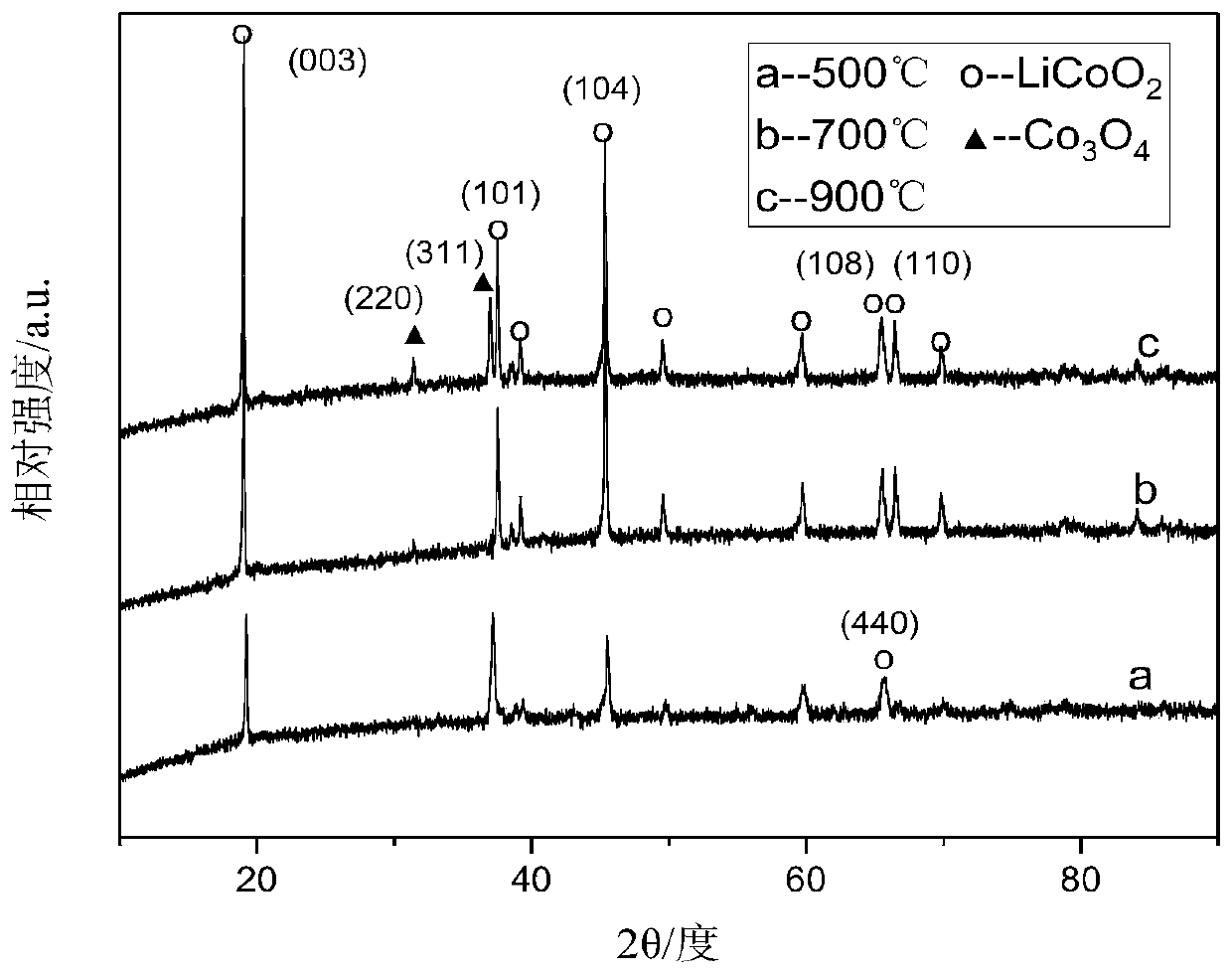
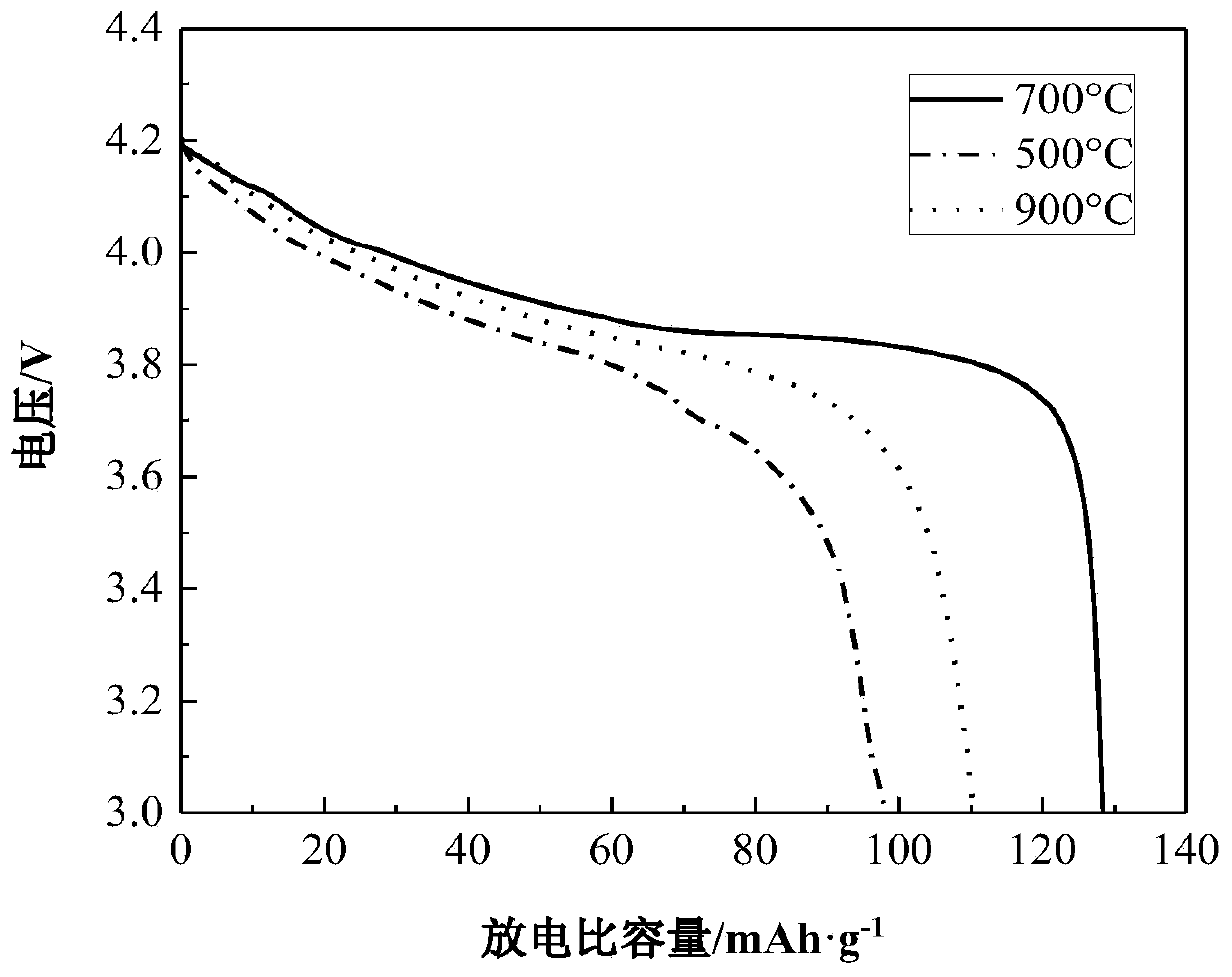
[0029] After the waste lithium cobalt oxide battery was discharged and disassembled, the positive electrode piece was put into the NMP solution for ultrasonication for 1 h. The stripped positive electrode material was calcined at 600° C. for 5 h to remove residual impurities in the positive electrode material. Get 5g of pretreated cathode material powder and add 250mL of citric acid (1mol / L) and H 2 o 2 (2vol%) mixed solution, stirred at 80°C for 60min, and then filtered to obtain the leachate. According to the Co in the leaching solution 2+ and Li + concentration, add corresponding cobalt source (basic cobalt carbonate) or lithium source (lithium hydroxide), and adjust the molar ratio of lithium and cobalt to 1:1. Reuse NH 3 ·H 2 O solution adjusted the pH of the leaching solution to 7, stirred at 80°C until a sol was formed, and then dried the sol in vacuum at 100°C for 24h to obtain a xerogel. The xerogel was calcined at 500 °C for 4 h, and finally, the calcined prod...
Embodiment 2
[0031] After the waste lithium cobalt oxide battery was discharged and disassembled, the positive electrode piece was put into the NMP solution for ultrasonication for 1 h. The stripped positive electrode material was calcined at 600° C. for 5 h to remove residual impurities in the positive electrode material. Get 5g of the pretreated cathode material powder and add 200mL of citric acid (1.5mol / L) and H 2 o 2 (3vol%) mixed solution, stirred at 70°C for 70min, and then filtered to obtain the leachate. According to the Co in the leaching solution 2+ and Li + concentration, add corresponding cobalt source (basic cobalt carbonate) or lithium source (lithium hydroxide), and adjust the molar ratio of lithium and cobalt to 1:1. Reuse NH 3 ·H 2 O solution adjusted the pH of the leaching solution to 7, stirred at 80°C until a sol was formed, and then dried the sol in vacuum at 100°C for 24h to obtain a xerogel. The xerogels were calcined at 700 °C for 4 h, and finally, the calci...
Embodiment 3
[0033] After the waste lithium cobalt oxide battery was discharged and disassembled, the positive electrode piece was put into the NMP solution for ultrasonication for 1 h. The stripped positive electrode material was calcined at 600° C. for 5 h to remove residual impurities in the positive electrode material. Get 5g of pretreated cathode material powder and add 330mL of citric acid (2mol / L) and H 2 o 2(1vol%) mixed solution, stirred at 60°C for 80min, and then filtered to obtain the leachate. According to the Co in the leaching solution 2+ and Li + concentration, add corresponding cobalt source (basic cobalt carbonate) or lithium source (lithium hydroxide), and adjust the molar ratio of lithium and cobalt to 1:1. Reuse NH 3 ·H 2 O solution adjusted the pH of the leaching solution to 7, stirred at 80°C until a sol was formed, and then dried the sol in vacuum at 100°C for 24h to obtain a xerogel. The xerogels were calcined at 900 °C for 4 h, and finally, the calcined pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com