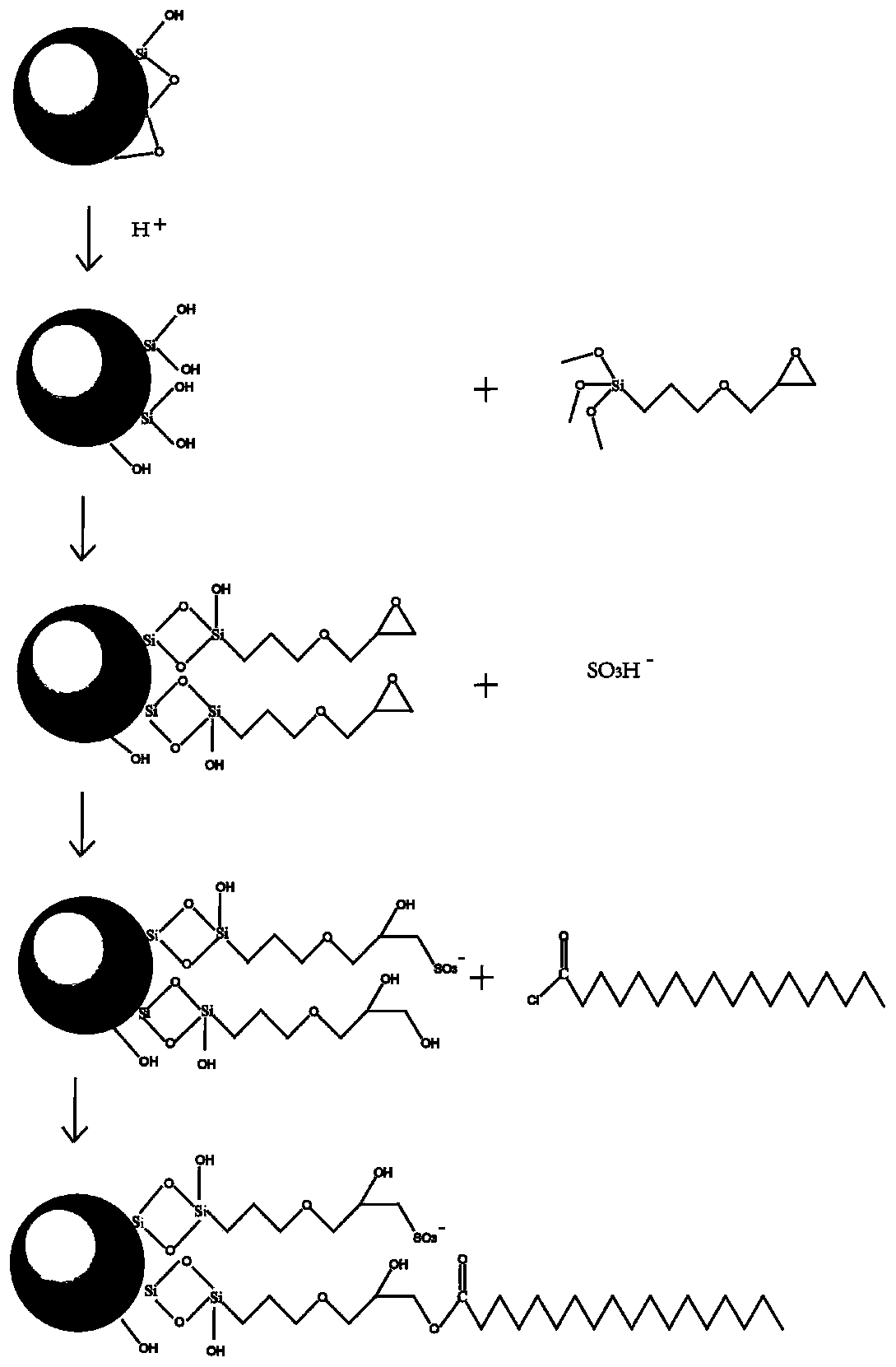
Reversed phase/ion exchange mixed mode chromatography stationary phase, preparation method and application
A mixed-mode chromatography and chromatographic stationary phase technology, applied in the field of reversed-phase/ion-exchange mixed-mode chromatography stationary phase, preparation and application, can solve the problems of few varieties, single structure, inability to adapt to complex components, etc. Harm, good separation effect, simple and feasible process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Preparation of activated silica gel: add a certain amount of silica gel particles to 8-10 times the mass of 10%-15% HCl solution, stir, reflux at 105°C for 8 hours, wash with water until neutral, and vacuum dry at 80°C.
[0048] Preparation of epoxy-based silica gel: Put the above-mentioned acidified silica gel particles into an autoclave with a Teflon liner, and add 1.92mmol-3.6mmol of γ-(2,3-epoxypropoxy)propyl trimethyl per gram Oxysilane, seal the autoclave, and react at 150°C for 8h. The unreacted silylating reagent was washed away with absolute ethanol, the product was collected by filtration, and vacuum-dried at 80° C. to obtain epoxy-functionalized porous silica gel.
[0049] Preparation of sulfonic acid-based silica gel: Disperse the above-mentioned epoxy-bonded silica gel microspheres into ultrapure water with a mass number of 8-10 times, add 1.92mmol-3.6mmol sodium bisulfite per gram, and ultrasonically mix. Adjust the pH value to 7-8 with triethylamine, rea...
Embodiment 1
[0066] Activation of embodiment 1 silica gel microspheres
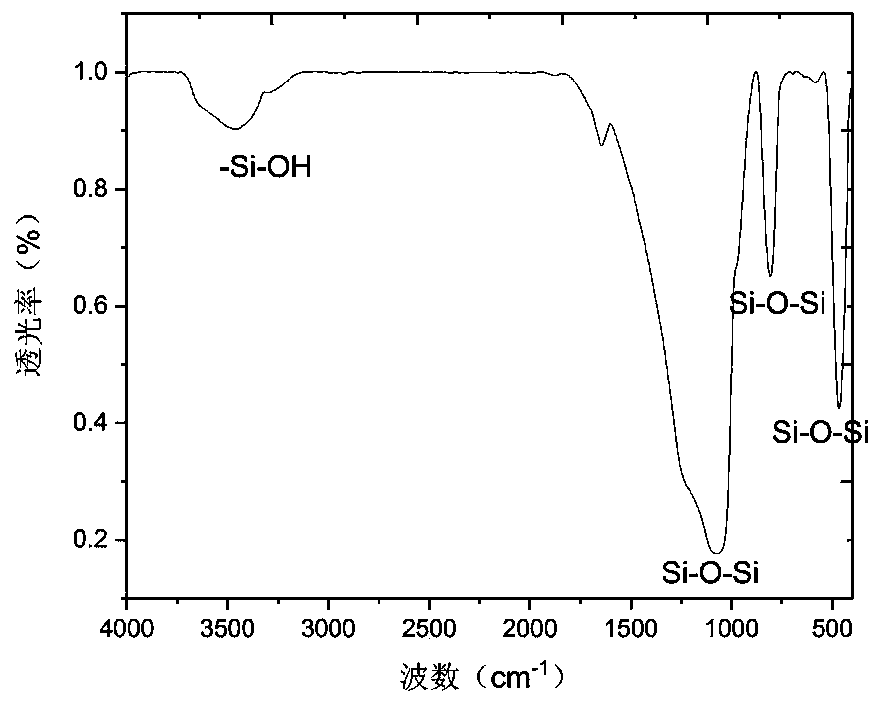
[0067] Take 10g of silica gel microspheres and add them to a 250mL three-neck flask, add 100mL of 10% HCl solution, stir mechanically, reflux at 105°C for 8h, wash with water until neutral, and dry in vacuum at 120°C overnight to obtain activated silica gel microspheres. The infrared characterization spectrum of silica gel microspheres is as follows: figure 2 shown at 3400cm -1 There are larger silanol absorption peaks.
Embodiment 2
[0068] Embodiment 2 Dry method preparation of epoxy-bonded silica gel microspheres
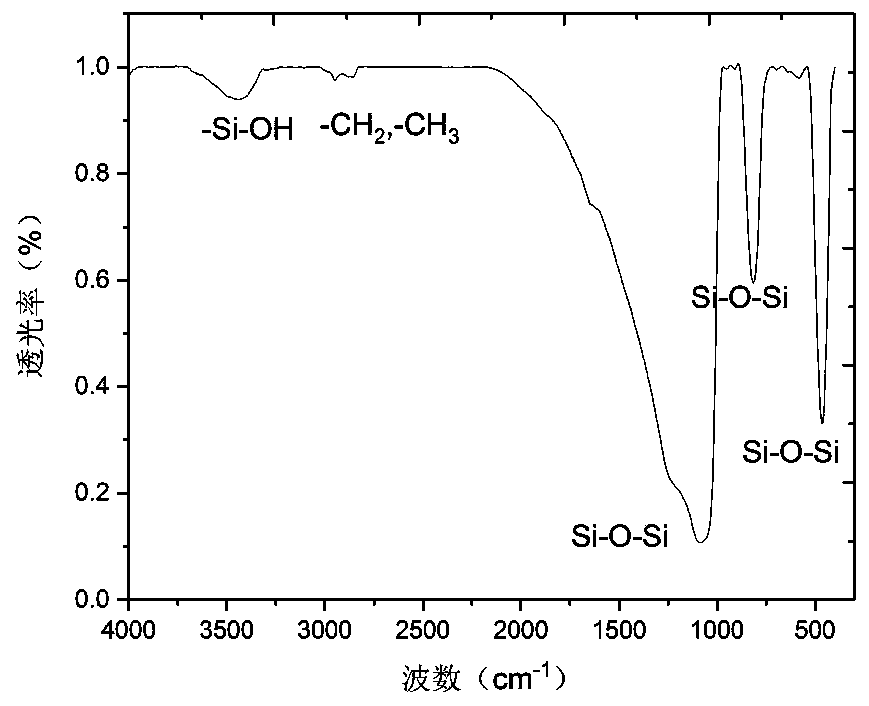
[0069] Take 10 g of activated silica gel microspheres prepared according to Example 1 and put them into an autoclave, add 5.31 mL of γ-(2,3-glycidoxy)propyltrimethoxysilane, and react at 150° C. for 8 h. Wash with absolute ethanol and dry under vacuum at 80°C to obtain epoxy-functionalized silica gel microspheres. The infrared characterization spectrum of the epoxy-functionalized silica gel is as follows: image 3 shown, after bond and epoxy at 2800cm -1 and 2890cm -1 The stretching vibration peak of C-H appears nearby, indicating that the epoxy group is bonded to the surface of the silica gel. The elemental analysis and characterization are shown in Table 1. The carbon content is 8.05%, and the calculated surface bond amount is 4.50mol / m 2 . The outstanding advantage of the method is that it does not use harmful organic solvents such as toluene, which is beneficial to the protection of he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com