Method for preparing arsenic trioxide by utilizing arsenic sulfide residues
A technology of arsenic trioxide and arsenic sulfide, which is applied in arsenic oxide/arsenic hydroxide/oxyacid arsenic, chemical instruments and methods, arsenic compounds, etc. The problems of high consumption and price, to achieve the effect of saving equipment investment and production costs, efficient extraction and recycling, and broad prospects for industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
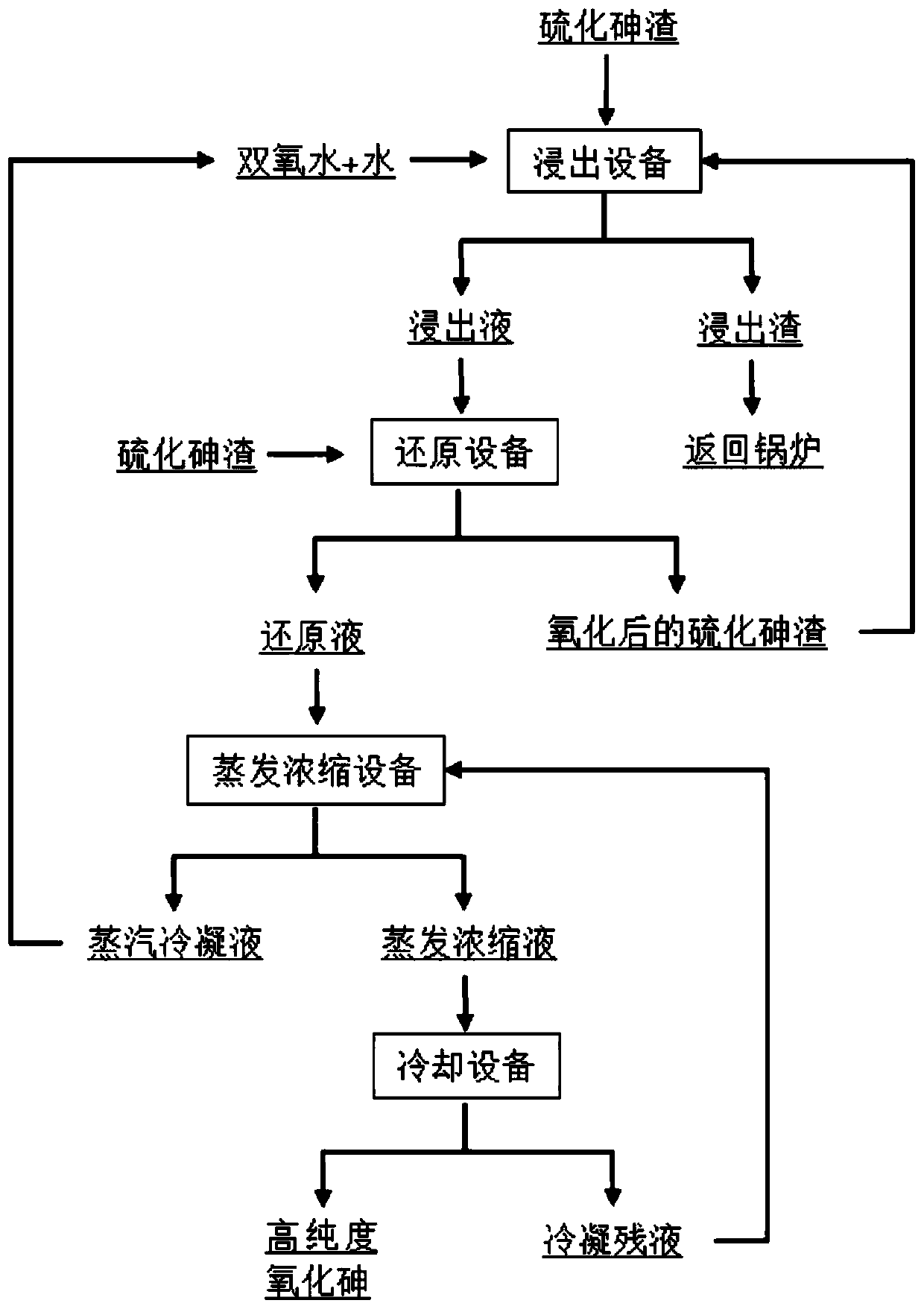
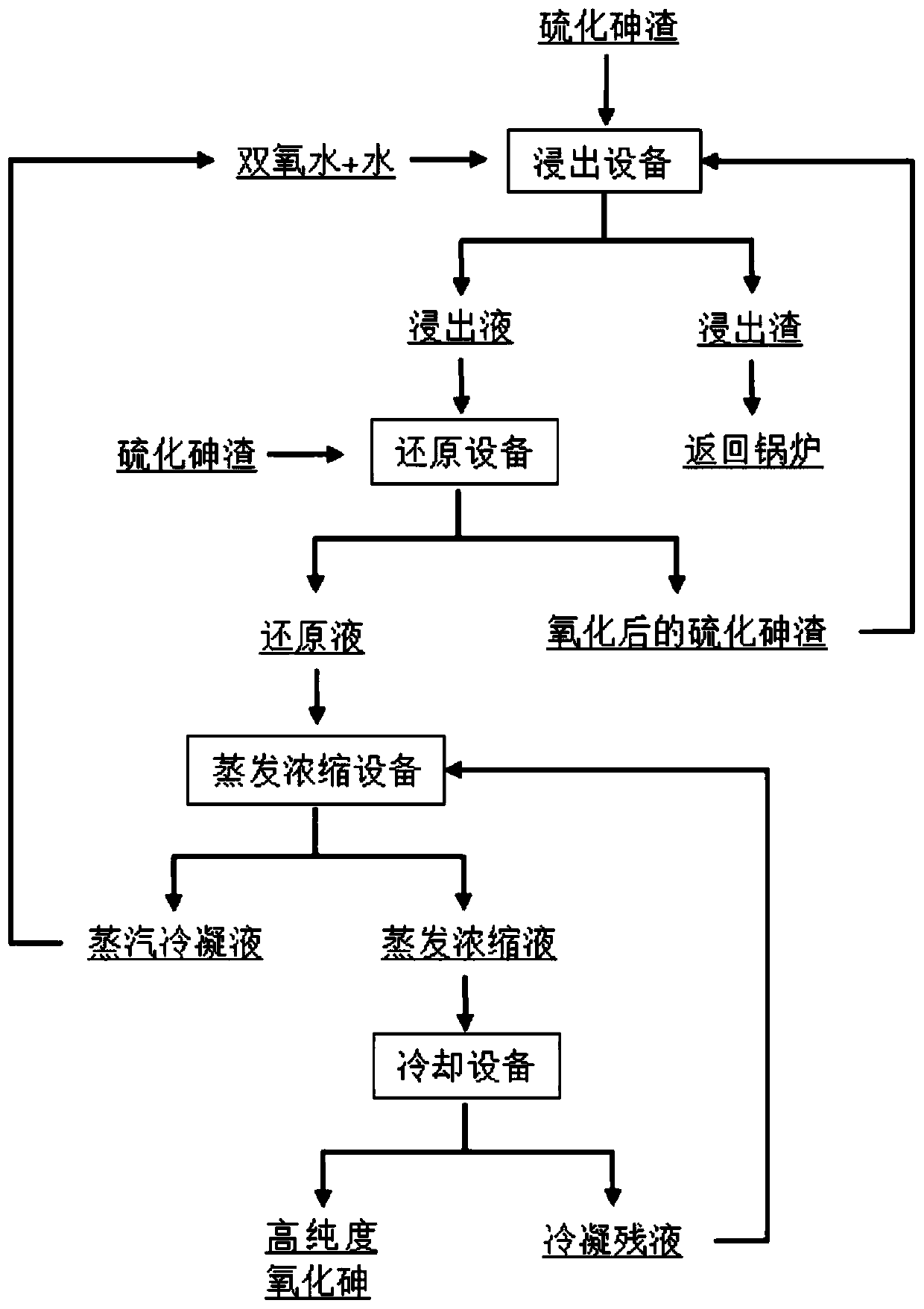
[0037] Embodiments of the present invention provide a method for preparing arsenic trioxide from arsenic sulfide slag, such as figure 1 As shown, it specifically includes the following steps:
[0038] (1) Fully mix the arsenic sulfide slag with a content of 40% and water, and the water consumption is calculated as 200ml / 100g (arsenic sulfide slag), and slowly add hydrogen peroxide (mass fraction is 30%) in a water bath at 60°C with stirring. , for oxidation, the oxidation time is 60min, 30% hydrogen peroxide is calculated as 300g / 100g arsenic sulfide slag, after the oxidation reaction is completed, the solution is filtered and the filtrate is collected, the arsenic content of the obtained filter residue is 98%. Pentavalent arsenic accounts for 92% of the total arsenic in the filtrate;
[0039](2) adding an equivalent mass ratio of 40% arsenic sulfide slag to the filtrate obtained in step (1), mixing fully, that is, the content of arsenic sulfide added in step (2) is equal to ...
Embodiment 2
[0042] The embodiment of the present invention provides a method for preparing arsenic trioxide by using arsenic sulfide slag, which specifically includes the following steps:
[0043] (1) Mix arsenic sulfide residue with 50% arsenic sulfide slag and water fully, the water consumption is calculated as 300ml / 100g arsenic sulfide slag, slowly add 30% hydrogen peroxide in a water bath at 80°C under agitation, and oxidize, and the oxidation time For 15 minutes, 30% hydrogen peroxide is calculated as 400g / 100g of arsenic sulfide slag. After the oxidation reaction is completed, the solution is filtered and the filtrate is collected. The arsenic content of the obtained filter residue is 98%. 95% of total arsenic;
[0044] (2) to step (1) gained filtrate, add the content of equivalent mass proportion to be 50% arsenic sulfide slag and mix fully, and water bath pan reduces reaction at 72 ℃, and the molar concentration of arsenic sulfide in the control reaction system is 0.3028mol / L, ...
Embodiment 3
[0047] The embodiment of the present invention provides a method for preparing arsenic trioxide by using arsenic sulfide slag, which specifically includes the following steps:
[0048] (1) Mix arsenic sulfide residue with 60% arsenic sulfide slag and water fully, the water consumption is calculated as 400ml / 100g arsenic sulfide slag, slowly add 30% hydrogen peroxide in a water bath at 70°C under agitation, and oxidize, and the oxidation time For 45 minutes, 30% hydrogen peroxide is calculated as 500g / 100g of arsenic sulfide slag. After the oxidation reaction is completed, the solution is filtered and the filtrate is collected. The arsenic content of the obtained filter residue is 98%. The pentavalent arsenic in the obtained filtrate accounts for 99% of total arsenic;
[0049] (2) Add the arsenic sulfide slag with the content of 60% to the filtrate obtained in step (1) and mix fully, and a reduction reaction occurs in a water bath at 90° C., and the reduction reaction time is 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com