Indenoanthracene derivative compound and application thereof
A technology of compounds and derivatives, applied in the field of organic electroluminescence functional materials and devices, to achieve the effect of improving luminescence stability and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
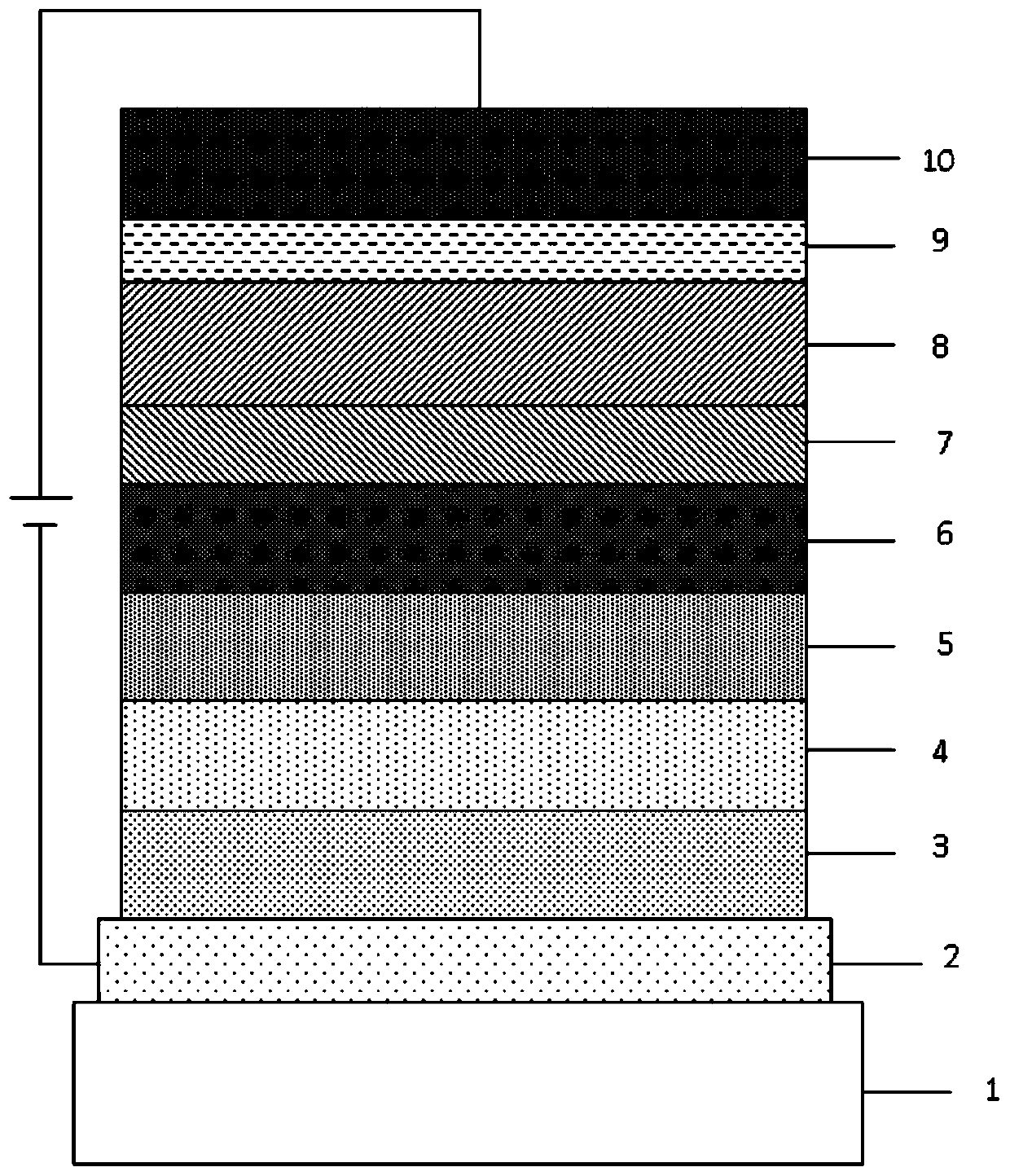
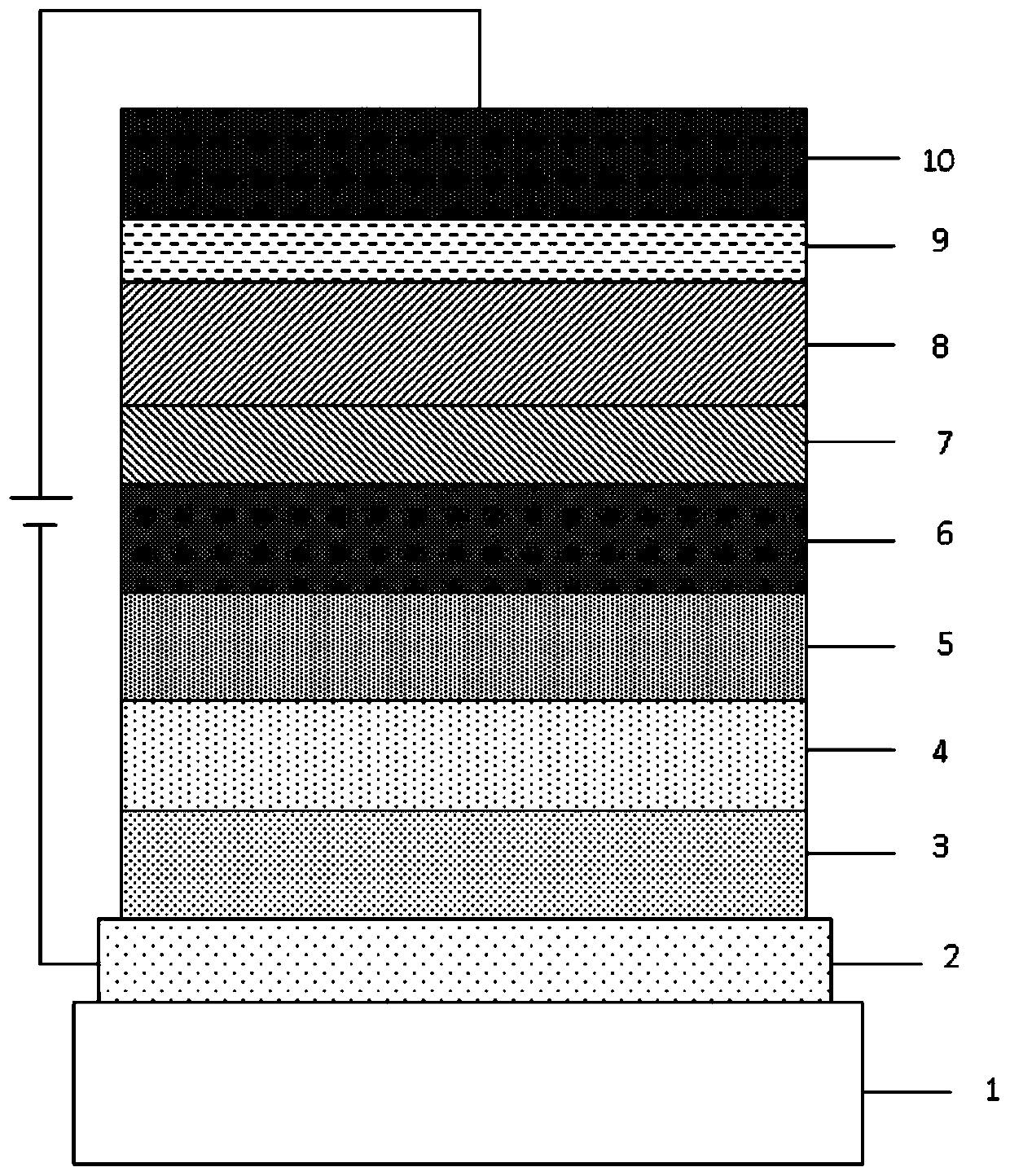
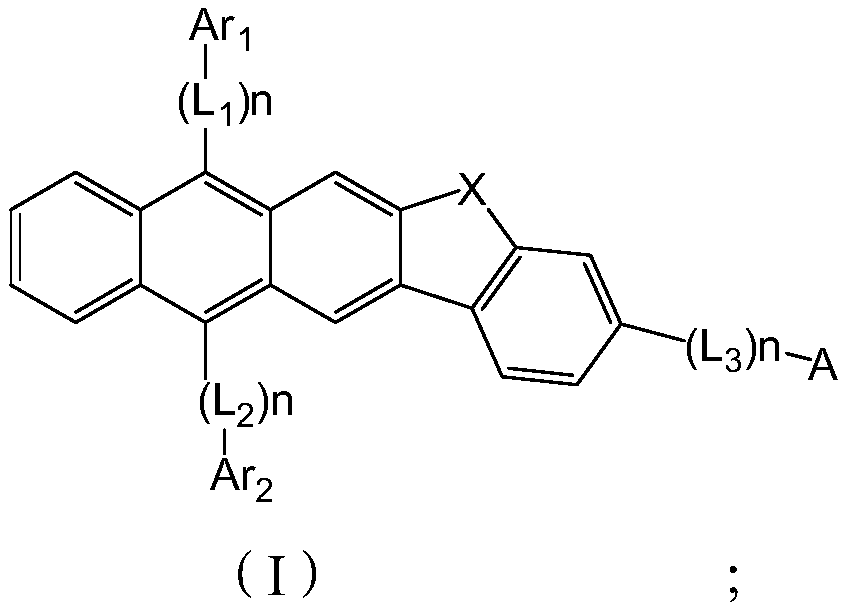
Image
Examples
Embodiment 1
[0133]
[0134] Introduce nitrogen into a 1L three-necked flask, add 12.8g carbazole, 20g intermediate 1-5, 16.9g potassium carbonate, 0.81g 18-crown-6 ether, 400ml DMF, stir until the raw materials are completely dissolved, then add 0.58g iodide Cuprous, heated to 130°C and stirred for 10 hours, TLC monitored the complete consumption of compound 1-5, stopped the reaction, poured the reaction solution into 3 times the volume of water after cooling down to room temperature, stirred to precipitate solid, filtered, washed the filter cake with water until neutral, After the filter cake was dried, it was dissolved in toluene, passed through an insulating column, and the eluent was concentrated and recrystallized to obtain 20.8 g of white solid, namely compound 1, with a yield of 82.3%.
[0135] NMR data of compound 1: 1 H NMR (400MHz, CDCl3) δ8.08 (d, J = 7.6, 1H), 7.67-7.71 (m, 4H), 7.52-7.55 (m, 6H), 7.46 (td, J = 7.6, 4H), 7.32 -7.40 (m, 12H), 7.08 (td, J=7.2, 4H), 7.00 (td,...
Embodiment 2
[0137]
[0138] Introduce nitrogen into a 1L three-necked flask, add 11.8g sodium tert-butoxide, 16.0g 9,9-dimethylacridine, 20g intermediate 1-5, 400ml toluene, stir until the raw materials are completely dissolved, then add 69mg palladium acetate , 0.3ml tri-tert-butylphosphine toluene solution, heated to reflux and stirred for 12 hours, TLC monitored the complete consumption of compound 1-5, stopped the reaction, filtered after cooling down to 60-70°C, washed the filtrate with hot water until neutral, refluxed to remove water After passing through the insulation column, the eluate was concentrated and recrystallized to obtain 21.9 g of white solid, namely compound 3, with a yield of 78.5%.
[0139] NMR spectrum data of compound 3: 1 H NMR (400MHz, CDCl3) δ8.08(d, J=7.6,1H), 7.68(d, J=7.2,4H), 7.52(td, J=7.6,2H), 7.46(td, J=7.6, 4H), 7.32-7.40(m, 8H), 6.88(d, J=7.2, 8H), 6.83(td, J=7.2, 4H), 6.54(td, J=7.2, 4H), 1.73(s, 6H ), 1.67(s,12H).
Embodiment 3
[0141]
[0142] The synthesis procedure is the same as that of intermediate 1-4, except that intermediate 1-3 is replaced by intermediate 5-1, the amount of butyllithium is 10.3ml, the amount of diphenylphosphine chloride is 5.2g, hydrogen peroxide The amount of used was 20ml, and 17.5g of compound 8 was obtained, with a yield of 76.8%.
[0143] NMR data of compound 8: 1 H NMR (400MHz, CDCl3) δ8.13 (d, J = 7.6, 1H), 7.83 (s, 1H), 7.65-7.68 (m, 3H), 7.52-7.61 (m, 12H), 7.40-7.46 (m , 8H), 7.32-7.35 (m, 10H), 7.08 (td, J = 7.2, 4H), 7.00 (td, J = 7.2, 4H), 1.73 (s, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com