Amino acid derivatization method and application thereof
An amino acid and derivatization technology, applied in the field of amino acid detection, can solve the problems of long derivatization time and cumbersome derivatization process, and achieve the effects of short derivatization time, simple and fast derivatization process, and reducing the influence of steric hindrance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
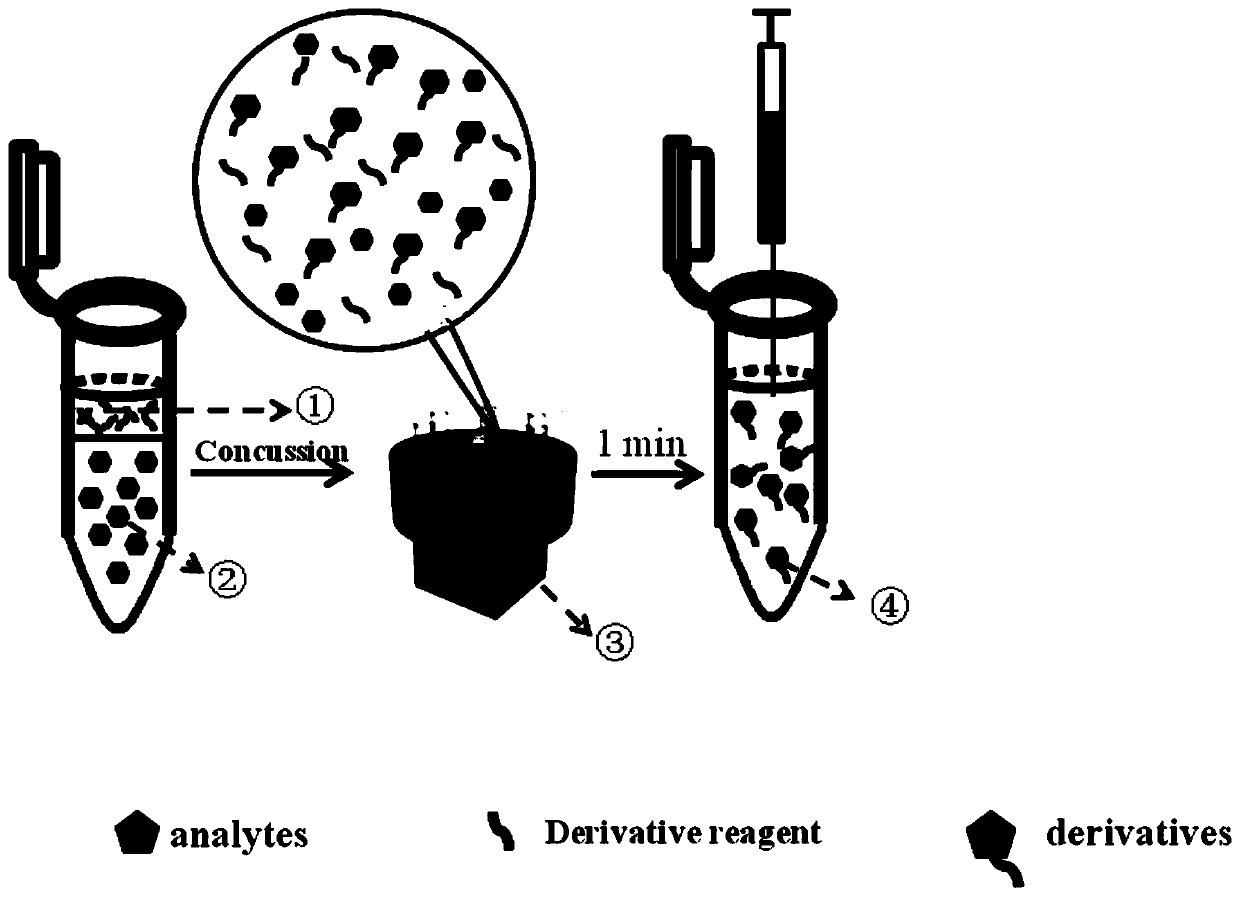
[0055] The amino acids in this example are 20 kinds of natural L-type amino acids, and the preparation method of amino acid derivatization is as follows:
[0056] (1) Preparation of 4-nitrobenzoyl chloride derivatization reagent:
[0057] Take 10 mg of 4-nitrobenzoyl chloride and dissolve it in a certain amount of anhydrous acetonitrile to prepare a 10 mg / L derivatization reagent.
[0058] (2)NH 4 HCO 3 -NH 4 Preparation of OH buffer solution:
[0059] Take 0.39g of NH 4 HCO 3 Dissolve in ultrapure water, add ammonia water, and dissolve in a 50mL volumetric flask to make the buffer solution pH=9.
[0060] (3) Preparation of amino acid standard solution:
[0061] Take 10mg amino acid standard, use 0.1mol / L NH 4 HCO 3 -NH 4 The buffer solution of OH was prepared into a mixed standard solution of 10μg / L for later use.
[0062] (4) Take 900 μL of 10 μg / L amino acid mixed standard solution, add 100 μL of 10 mg / L 4-nitrobenzoyl chloride derivatization reagent, shake the reaction for 1 min, and ...
Embodiment 2
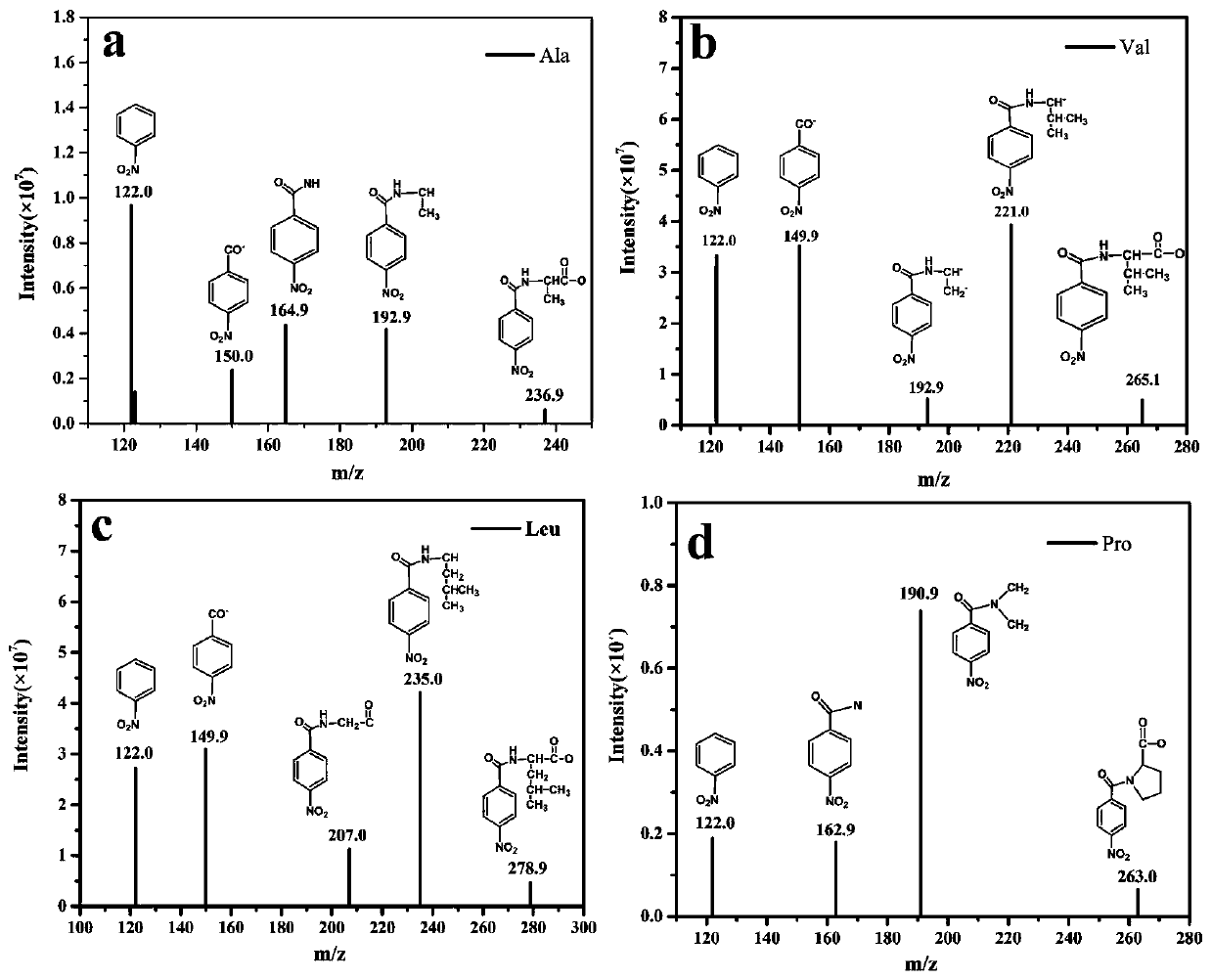
[0064] The derivatized amino acid prepared in Example 1 was sampled and analyzed, and the parent ion and product ion scanning analysis of the ultra-high performance liquid chromatography-triple quadrupole mass spectrometry was performed.
[0065] Chromatographic column: Shim-pack XR-ODS III (2.2μm, 2.0mmi.d.×200mm); injection volume: 20μL; column temperature: 35℃; elution method: gradient elution: mobile phase A: 0.1% An aqueous solution of formic acid, mobile phase B: acetonitrile; flow rate: 0.3 mL / min; time: 20 min. The detection conditions for triple quadrupole mass spectrometry are: ion source: ESI; detection method: multi-reaction monitoring mode; capillary voltage: 3.0kV; ion source temperature: 350°C; desolventizing gas temperature: 400°C; desolventizing gas flow: 650L / hr; Cone air flow: 50L / hr.
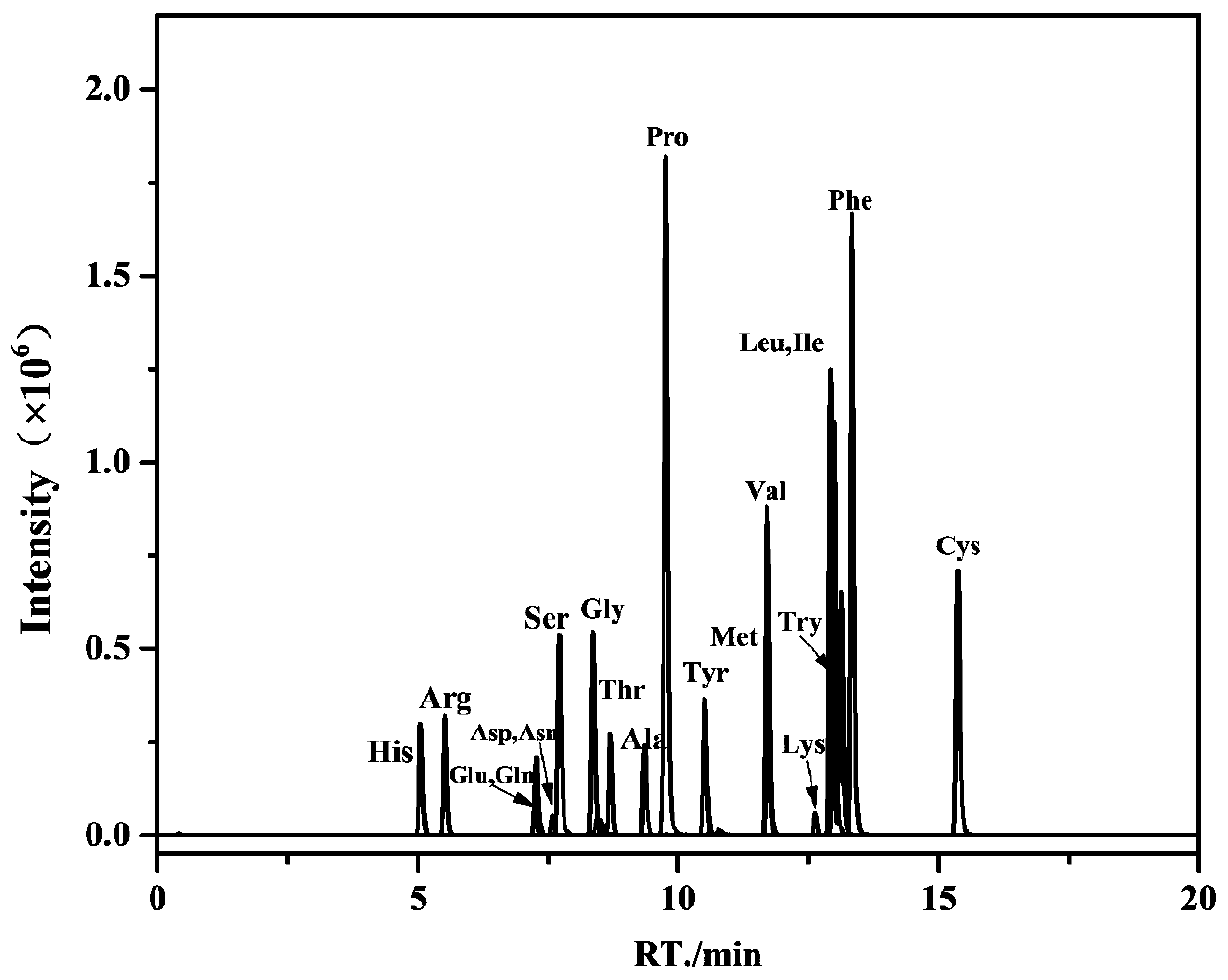
[0066] The derivatized amino acid prepared in Example 1 is tested by liquid chromatography and the results are as follows Figure 4 As shown, all 20 amino acids were detected. ...
Embodiment 3
[0076] Determination of amino acid content in tobacco leaf samples:
[0077] 1. Process the tobacco leaf samples according to the requirements of YC / T31-1996, accurately weigh 100mg tobacco leaf samples, add ultrapure water for extraction for 10 minutes, and filter the supernatant obtained after centrifugation with a 0.22μm aqueous phase filter membrane to obtain the extract . Take 100μL extract, use 0.1mol / L NH 4 HCO 3 -NH 3 The buffer solution is diluted to 10mL of the sample to be tested. Take 900μL of sample diluent after centrifugation, add 100μL of 10mg / L 4-nitrobenzoyl chloride derivatization reagent, shake the reaction for 1min, filter by organic filter and combine with liquid chromatography-triple quadrupole mass spectrometry. The MRM multi-channel reaction monitoring method is used for quantitative analysis. The amino acid content and standard addition experiment of tobacco leaf samples are shown in Table 3.
[0078] Table 3 Amino acid content, standard addition recover...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com