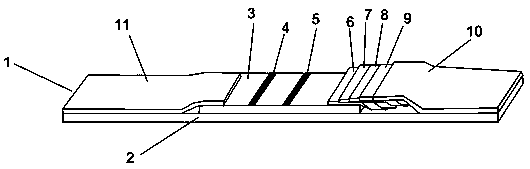
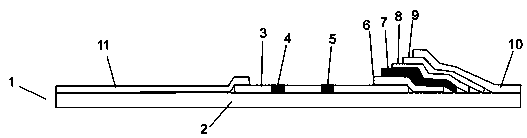
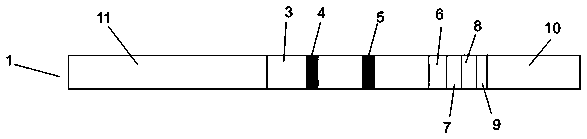
African swine fever virus detection test paper, kit and preparation method thereof
A technology for detection of African swine fever virus and test strips, which is applied in the field of detection test strips, kits and preparations of African swine fever virus, can solve the problems of insufficient sensitivity, short reaction time, complex sample components, etc., and achieve high detection sensitivity, The overall time-consuming is short and the effect of monodispersity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of monoclonal antibody against p72 recombinant protein of African swine fever virus
[0056] 1. African swine fever virus p72 recombinant protein gene cloning and recombinant expression vector construction
[0057] According to the African swine fever virus p72 gene sequence (sequence number: AAT84439.1) published by Genbank as a reference, the optimized gene sequence was designed to make the N / C-terminal epitope of the antigen more easily exposed, showing more advantages. Therefore, a more suitable monoclonal antibody is screened. The present invention analyzes the dominant epitope region of the protein sequence through DNAstar and IEDB databases, and uses GGGGS as the linker to express the two main epitope regions in tandem, and the two epitopes The amino acid sequences of the regions are SEQ ID No. 1 and SEQ ID No. 2, which retain more amino acids while minimizing the impact of steric hindrance. The optimized nucleotide sequence of the African swine...
Embodiment 2
[0098] Example 2 Preparation of monoclonal antibody against African swine fever virus p72 recombinant protein labeled with latex microspheres
[0099] The prepared African swine fever virus p72 recombinant protein monoclonal antibody (7A7) is diluted with the diluent and labeled with latex microsphere particles. The specific operation is as follows:
[0100] 1. Cleaning of latex microspheres: Measure a certain volume of latex microspheres with a particle size of 300nm (purchased from Suzhou Weidu Biotechnology Co., Ltd., item number DR05C), pour it into a clean centrifuge tube, and add each 100 μL latex microspheres Add the labeling buffer to the ratio of 900 μL labeling buffer (50 mM MES, pH 6.0). Centrifuge at 17000 r / min for 10 min, remove the supernatant, add 1000 μL of labeling buffer, centrifuge at 17000 r / min for 10 min, remove the supernatant, and add 1000 μL of labeling buffer to resuspend the microspheres.
[0101] 2. Activation of latex microspheres: Weigh 20 mg of NHS an...
Embodiment 3
[0104] Example 3 Preparation of latex microsphere pad
[0105] 1. Take out the latex microsphere labeled African swine fever virus p72 recombinant protein monoclonal antibody solution from the refrigerator at 4°C and return to room temperature.
[0106] 2. Take a piece of 20cm×30cm glass fiber and cut it into 0.6cm×30 cm size with an accessory cutting machine.
[0107] 3. Spread a layer of cling film on the table, place the cut glass fiber on the cling film, and use a pipette to suck 1 mL of the latex microsphere labeled African swine fever virus p72 recombinant protein monoclonal antibody solution to evenly moisten it A cut glass fiber. Dry at room temperature for 12 to 16 hours, then transfer to a 40°C oven for 1 hour, then place in a sealed bag with desiccant, seal, and set aside.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com