A kind of aryl silicon organic photoelectric material and its preparation method and application
An organic optoelectronic material, aryl technology, applied in the direction of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of scarcity of high-efficiency dark blue PhOLEDs, etc., achieve simple preparation and synthesis process, low start-up voltage, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
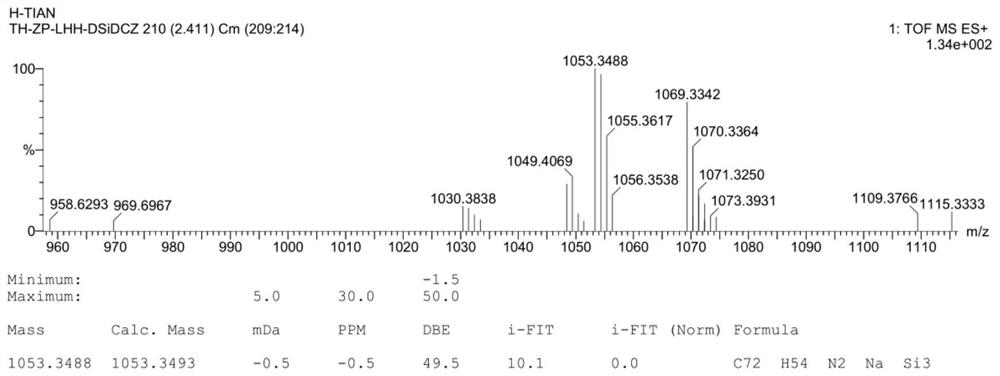
[0026] The preparation method of the aryl silicon organic photoelectric material DSiDCzSi provided by the invention comprises the following steps:
[0027] Step S1: Under the protection of nitrogen, first dissolve 3-bromo-9H carbazole in anhydrous tetrahydrofuran, and then add n-butyllithium to react for 1h-1.2h at a low temperature of 0°C to prepare a reaction system, in which 3-bromo The molar ratio of -9H carbazole to n-butyllithium is 1:1-1.5;
[0028] Step S2: Add diphenyldichlorosilane to the reaction system of step S1, and react at a low temperature of 0°C for 1h-1.2h, wherein the molar ratio of 3-bromo-9H carbazole to diphenyldichlorosilane is 2- 2.5:1, then warmed up to room temperature to continue the reaction for 12 hours, extracted and purified to obtain the compound bis(3-bromo-9H-carbazol-9-yl)diphenylsilane;
[0029] Step S3: Under nitrogen protection, first dissolve the bis(3-bromo-9H-carbazol-9-yl)diphenylsilane prepared in step S2 in anhydrous tetrahydrofura...
Embodiment
[0032] Take a single-necked flask, add 3.0g of 3-bromo-9H carbazole, seal it and pump nitrogen repeatedly three times, inject and dissolve 40mL of anhydrous tetrahydrofuran (THF) under a nitrogen atmosphere, and then put it into an ice-cold flask at 0°C / Cool in a water bath for 15 minutes. Measure 8.3 mL of n-butyl lithium in n-hexane solution (1.6 M) with a syringe, add it dropwise into the reaction bottle, and react at 0° C. for 1.0 h to obtain a reaction system. Add 1.1 mL of diphenyldichlorosilane to the reaction system, react at 0° C. for 1.0 h, and then rise to room temperature for 12 h. The reaction solution was quenched with 50 mL of water, extracted with 3×200 mL of dichloromethane solution, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50mL of dichloromethane, added silica gel powder and then spin-dried the solvent, and purifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
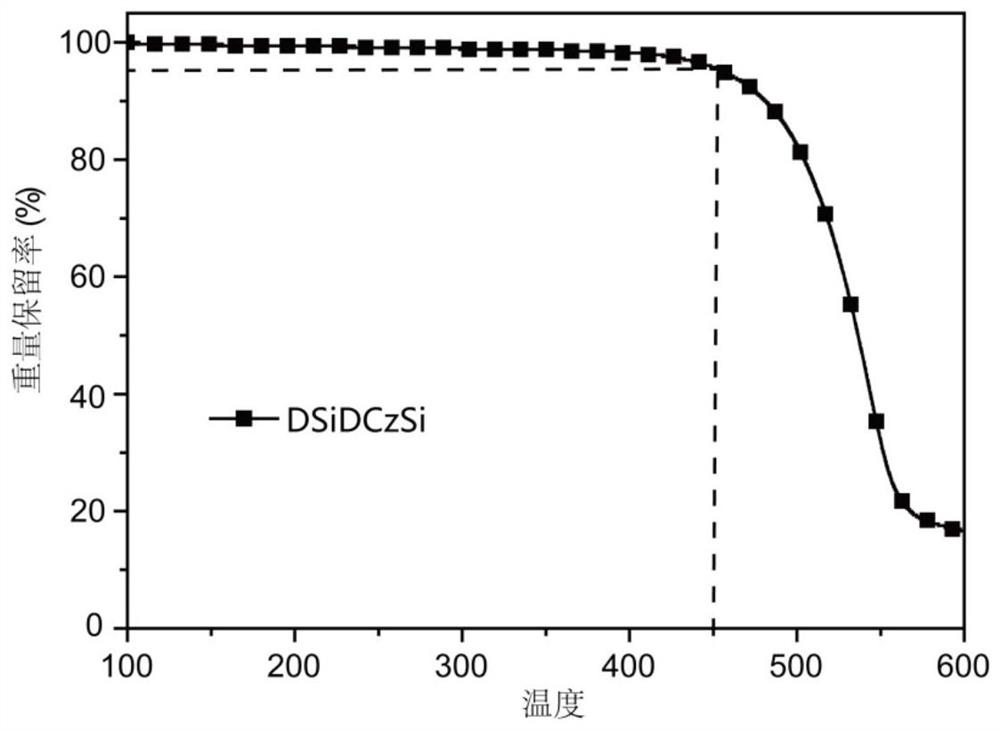
| thermal decomposition temperature | aaaaa | aaaaa |
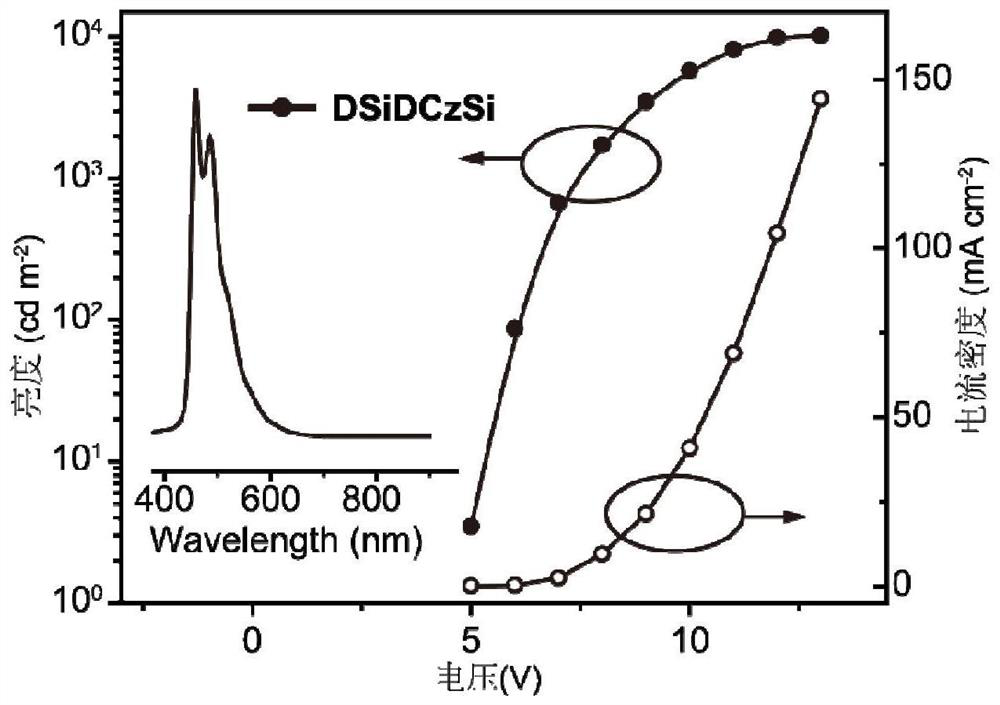
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com