Prodrug with tumor targeting and preparation method and application of prodrug
A technology of tumor-targeting and prodrugs, which is applied in the field of tumor-targeting prodrugs and their preparation, can solve the problems that no precedents for cancer-targeted drug regimens have been found, and achieve increased tumor cell selectivity, Good tumor targeting and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
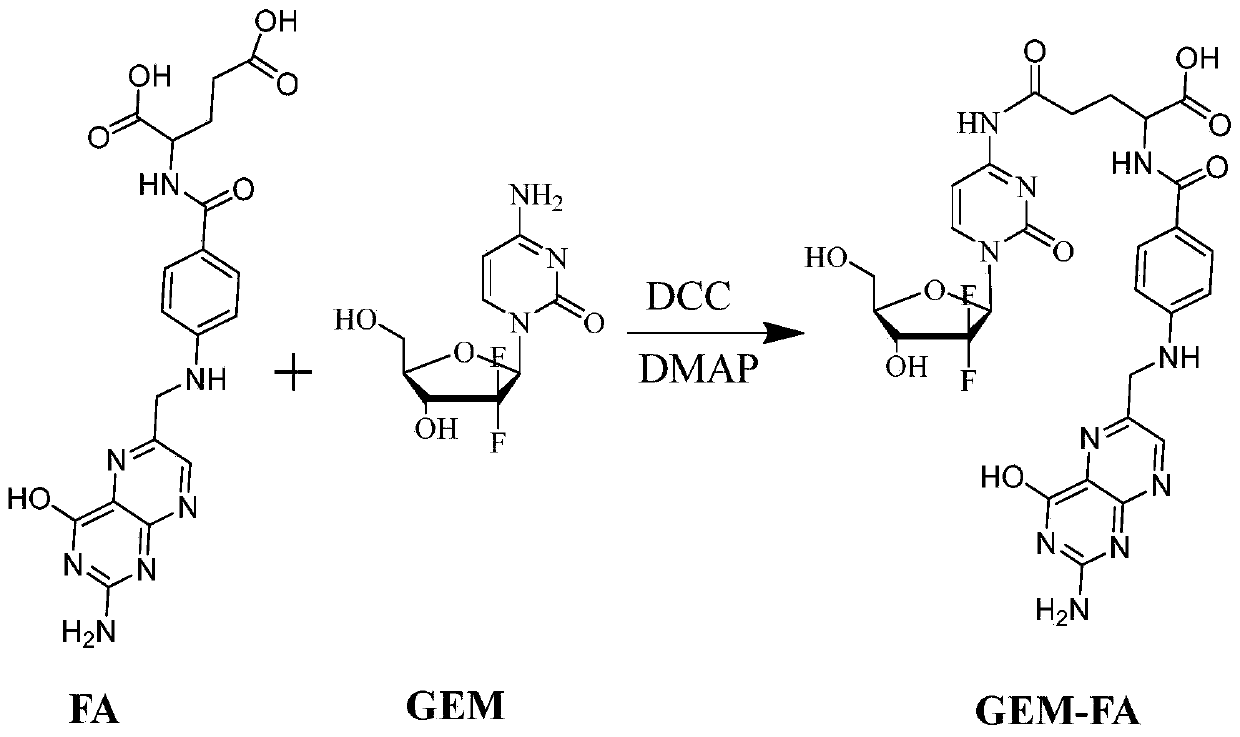
[0039] Example 1: Preparation of folic acid-gemcitabine prodrug
[0040] Take folic acid (0.15mmol) and dissolve it in 60ml DMSO. Under the action of dehydrating agent DCC (3.0mmol) and catalyst DMAP (3.0mmol), the reaction is stirred at 0℃ for 6h; then gemcitabine is added according to the molar ratio of folic acid 1:3 (Gemcitabine is Dissolved in DMSO), raise the temperature from an ice bath to room temperature 25℃, stir overnight in the dark, filter to remove by-products, concentrate the filtrate, recrystallize with ice ether or isopropanol, and purify by chromatography or preparative liquid phase , Freeze-drying can obtain tumor-targeting prodrug GEM-FA, C 28 H 28 F 2 N 10 O 9 , The theoretical MW is 686.5 (the yield is about 70%), and the ion peak MS is identified by mass spectrometry + Is 687.6. The structure characterization data are as follows, and the structure is determined as shown in formula (I):
[0041] 1 H-NMR (300MHz, DMSO): δ 2.05-2.14 (m, 4H), 3.55-3.61 (m, 2H), ...
Embodiment 2
[0042] Example 2: Preparation of folic acid-gemcitabine prodrug
[0043] 1) Preparation of folic acid active ester (γ-NHS-FA): Dissolve folic acid (1.0g, 2.3mmol) in 40ml anhydrous DMSO, add 0.5ml TEA triethylamine and mix and stir in anhydrous environment at room temperature and avoid light Overnight; then mix 0.47g (2.3mmol) of DCC and 0.26g (2.3mmol) of NHS, stir for 24h in the dark, filter to remove by-product dicyclohexylurea, and vacuum dry at low temperature to remove DMSO and TEA to obtain active folate.
[0044] 2) Preparation of folic acid-gemcitabine prodrug: Dissolve folic acid active ester γ-NHS-FA (0.15mmol) in 60ml of anhydrous DMSO / TEA (volume ratio 2:1), take it with γ-NHS-FA, etc. Add moles of gemcitabine to the mixed solution and react overnight in the absence of light under anhydrous conditions. The reaction solution is vacuum dried, recrystallized with ice ether or isopropanol, purified by chromatography or liquid phase preparation, and lyophilized to obtain tu...
Embodiment 3
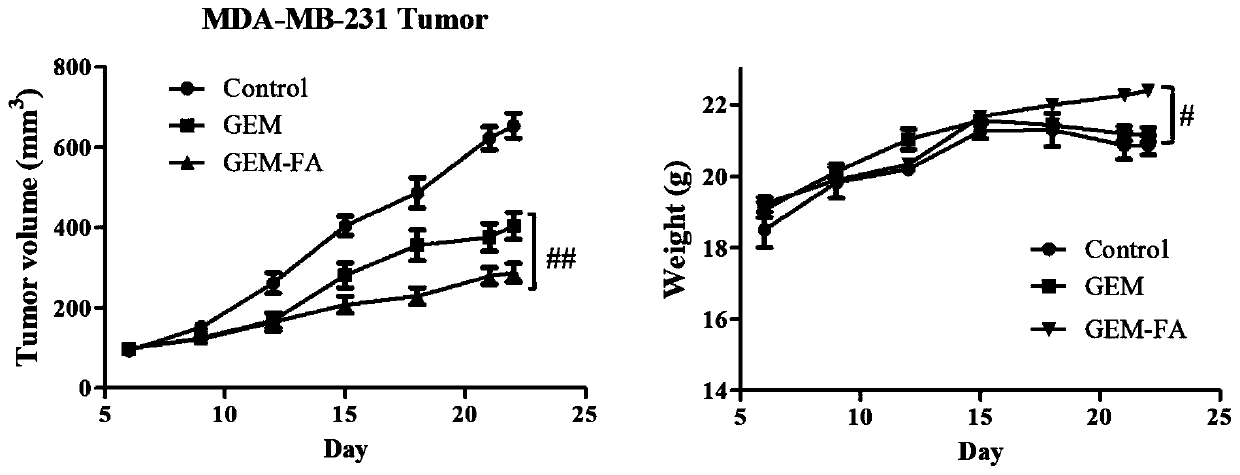
[0046] Example 3: Cytotoxicity detection and evaluation experiment
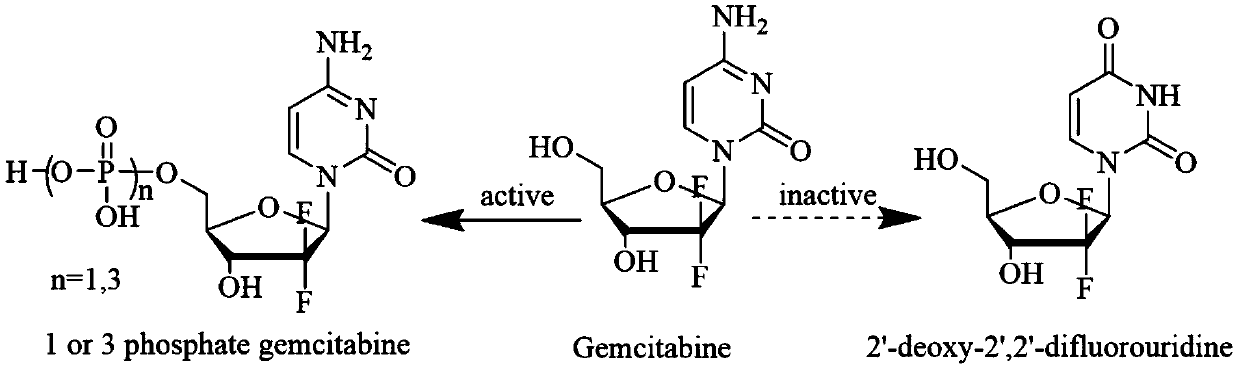
[0047] Evaluation and comparison of in vitro anticancer effects of GEM and GEM-FA. In view of the fact that gemcitabine nucleoside drugs are broad-spectrum anti-cancer toxicants, tumor cells (HeLa, HepG2, BXPC-3, MDA-MB-231 and SK- OV-3) The pharmacodynamic evaluation of the corresponding conjugate product prodrug GEM-FA and its prototype compound prepared in Example 1 was carried out, and the LO2 liver cells were subjected to the normal cell toxicity test.
[0048] Take the cells in the logarithmic growth phase and inoculate 2~10×10 according to the cell size 3 After growing on a 96-well plate, discard the supernatant, and then administer according to the following groups: cancer cells are divided into non-drug group and drug-added group (concentration of 0.05-50μM for cancer cells, concentration of 0.5-100μM for LO2 cells ; Where the concentration of folic acid is set to 0.5-100 μM), gemcitabine is used as a po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com