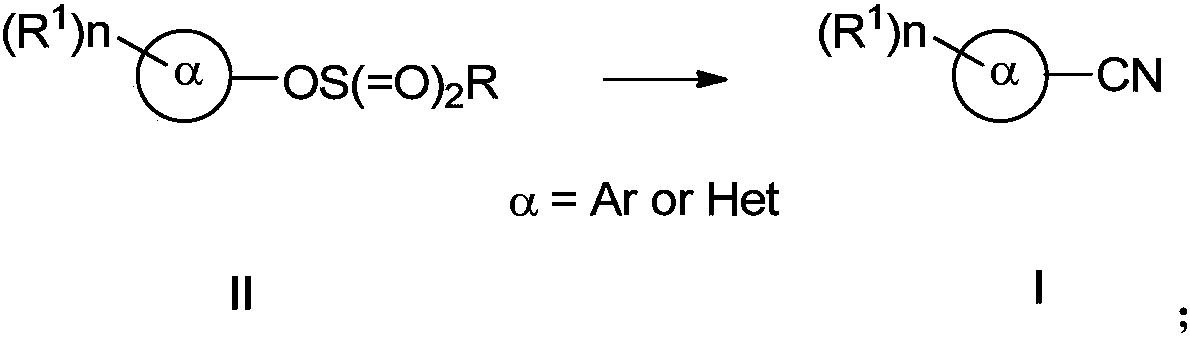
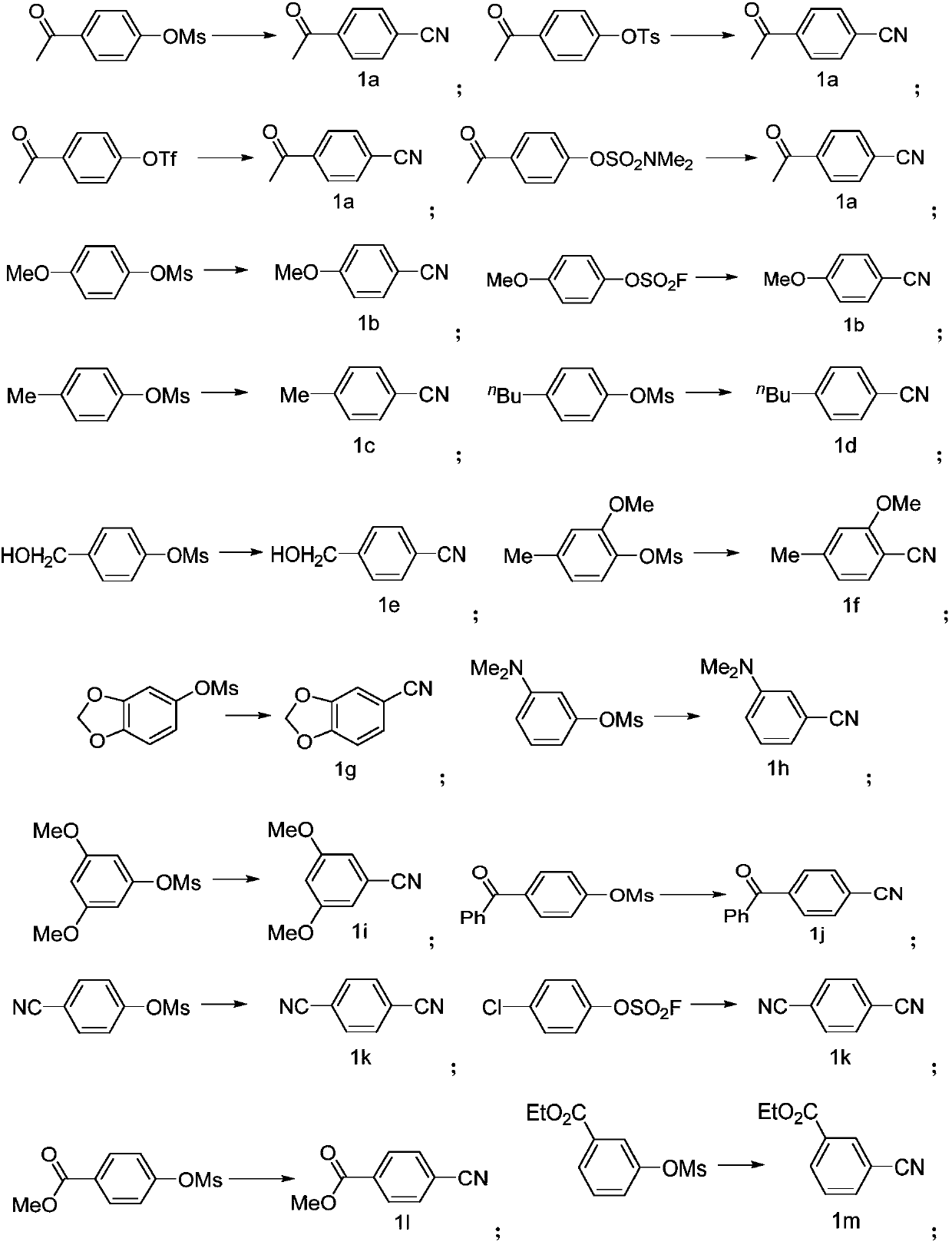
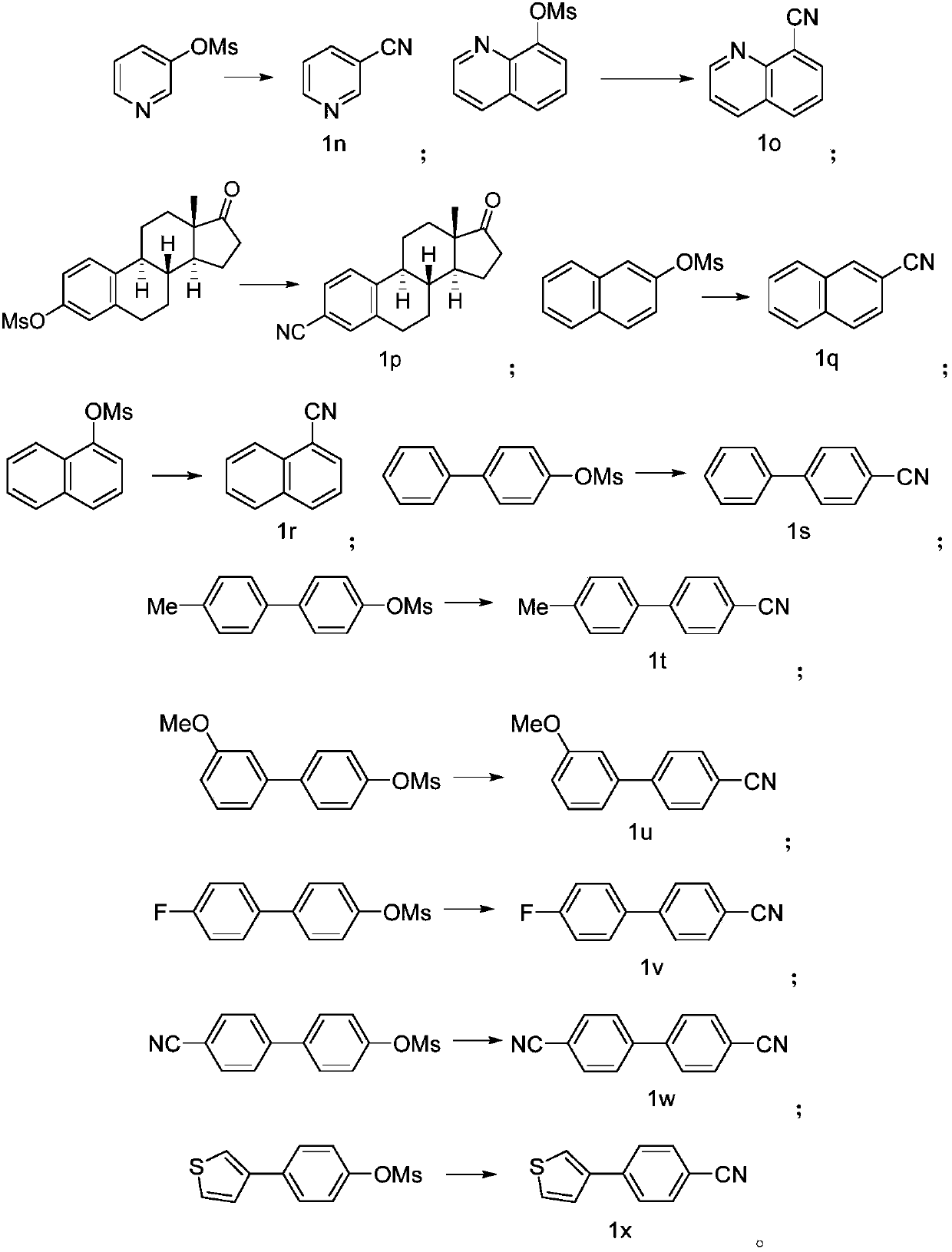
Preparing method of aromatic nitrile or alkenyl nitrile compound
A compound, aromatic nitrile technology, applied in the field of preparation of aromatic nitrile or alkenyl nitrile compounds, can solve the problems of poor substrate universality, expensive catalysts and ligands, poor functional group compatibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0099]
[0100] General preparation process: add p-iodophenol (1equiv), boric acid (1.2equiv), 5% Pd / CaCO to the round bottom bottle 3 (1.5mol%), K 2 CO 3 (2equiv), EtOH and water are dissolved, heated to 80°C under reflux, and TLC detects the reaction. After returning to room temperature, celite was filtered to remove the precipitate, the reaction was quenched with saturated brine, extracted with ethyl acetate, the organic phase was washed with water, washed with saturated brine, dried with anhydrous magnesium sulfate, filtered and concentrated, and then separated and purified.
[0101]
[0102] Silica gel column chromatography, eluent: petroleum ether / ethyl acetate / dichloromethane=8:1:1 to pure dichloromethane, the product is a white solid 1.81g, the yield is 91%. m.p=81.0-82.9℃. 1 H NMR(400MHz, CDCl 3 ): δ3.85(s,3H), 5.47(brs,1H), 6.84-6.89(m,3H), 7.07(s,1H), 7.12(d,J=7.6Hz,1H), 7.32(dd, J = 8.0 Hz, 1H), 7.45 (d, J = 7.6 Hz, 2H). 13 C NMR(100MHz, CDCl 3 ): δ55.30,112.07,112.48...
preparation example 2
[0106]
[0107] Phenol (1equiv) in a round bottom bottle is dissolved in ethyl acetate, and Et is added under ice water bath 3 After adding N(2equiv), MsCl(1.3equiv), remove the ice-water bath and react at room temperature. TLC detects that the reaction is complete. The reaction was quenched by adding water, extracted with ethyl acetate, the organic phase was washed with water, dried over anhydrous magnesium sulfate, filtered through silica gel, and the filtrate was concentrated and separated and purified.
[0108]
[0109] Silica gel column chromatography, eluent: petroleum ether / ethyl acetate=4:1, the product is light yellow liquid, the yield is 98%. 1 HNMR(400MHz, CDCl 3 ): δ0.93(t,J=7.2Hz,3H),1.32-1.38(m,2H),1.55-1.63(m,2H),2.61(t,J=7.6Hz,2H),3.11(s, 3H), 7.17-7.26 (m, 4H). 13 C NMR(100MHz, CDCl 3 ): δ13.83,22.21,33.44,34.94,37.09,121.63,129.79,142.28,147.18.IR(neat):3029,2957,2932,2860,1503,1365,1330,1197,1173,1147,1113,1018 ,968,865,841,814,776,740,681.HRMS(ESI)calcd for C ...
preparation example 3
[0121]
[0122] P-Aldehyde phenol (1.22g, 10mmol) in a round bottom bottle was dissolved in 30mL ethyl acetate, and Et was added at 0℃ 3 N (2.8 mL, 20 mmol), MsCl (1.0 mL, 13 mmol), the ice-water bath was removed after the addition, and the reaction was carried out at room temperature. TLC detected that the reaction was complete. Add water to quench the reaction, extract with ethyl acetate, wash the organic phase with water, wash with saturated brine, dry with anhydrous magnesium sulfate, filter with silica gel, concentrate the filtrate and proceed to the next reaction.
[0123] Dissolve the product from the previous step in 20mL MeOH and 20mL DCM, add NaBH in batches 4 (491.8mg, 13mmol). After the addition, the reaction was carried out at room temperature. TLC detected that the reaction was complete. Add saturated ammonium chloride to quench the reaction, extract with DCM, wash the organic phase with water, wash with saturated brine, dry with anhydrous sodium sulfate, filter and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com