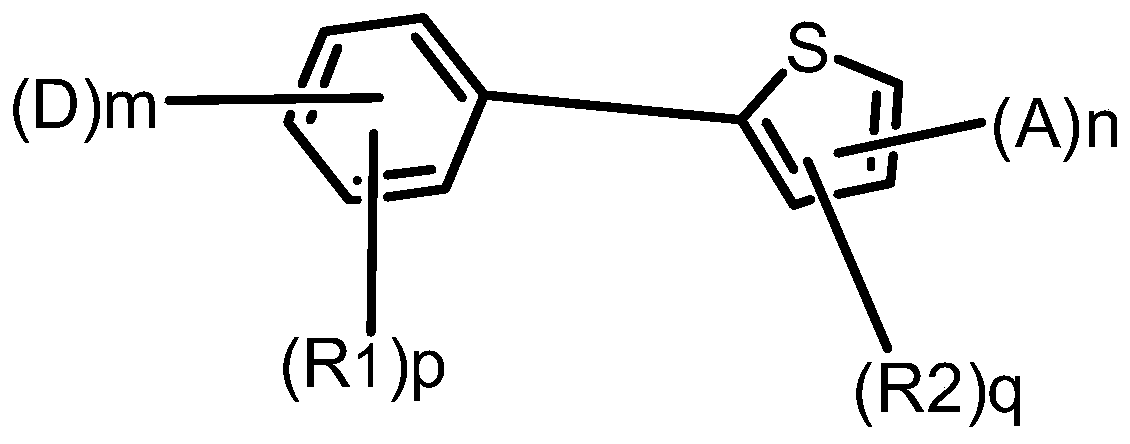
Compound, display panel and display device
A technology of compounds and derivatives, applied in the field of organic electroluminescent materials, to achieve the effects of high-efficiency energy transfer, high triplet energy level, and reduced conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114]
[0115] In a 250ml three-necked flask, first mix S1 (30mmol), pinacol diborate (36mmol), (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium(II) (0.3mmol) And potassium acetate (75mmol) was added separately, while stirring, degassing and nitrogen replacement were repeated 3 times quickly, and 100mL tetrahydrofuran was added through a syringe. Stir at a certain speed, heat the resulting mixed solution reactant to reflux at a reaction temperature of 80°C for 5 hours; after the reaction is completed, cool to room temperature and add 100ml of water, extract with ether, and dry the obtained organic phase with anhydrous sodium sulfate , the solvent was distilled off, and purified by column chromatography to obtain intermediate S2 (24.6 mmol, 82%).
[0116] MALDI-TOF MS: Calculated m / z: C 17 h 17 BFNO 2 S: 329.1; Measured: 329.5.
[0117]
[0118] Under nitrogen protection, weigh compound S2 (25mmol), S3 (25mmol), [Pd 2 (dba) 3 ]·CHCl 3 (0.5mmol) and HP(tBu) ...
Embodiment 2
[0125]
[0126] S6 (10.0mmol), S5 (10.5mmol), (dibenzylideneacetone) dipalladium (0) (0.5mmol), sodium tert-butoxide (14mmol), tri-tert-butylphosphine tetrafluoroborate (1.0mmol) Put it into a 500mL three-neck flask, and while stirring, quickly repeat degassing and nitrogen replacement 3 times, and add 200mL of toluene through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate S7 (8.6 mmol, 86%).
[0127] MALDI-TOF MS: m / z, Calculated: C 28 h 17 BrN 4 S: 520.0; test value: 520.4.
[0128]
[0129] In a 250ml three-necked flask, first, S7 (15.0mmol), pinacol diborate (18mmol), (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium(II...
Embodiment 3
[0136]
[0137] S6 (5.0mmol), S5 (5.2mmol), (dibenzylideneacetone) dipalladium (0) (0.25mmol), sodium tert-butoxide (8mmol), tri-tert-butylphosphine tetrafluoroborate (0.5mmol) Put it into a 250mL three-neck flask, and while stirring, quickly repeat degassing and nitrogen replacement 3 times, and add 80mL of toluene through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate S12 (3.85 mmol, 77%).
[0138] MALDI-TOF MS: m / z, Calculated: C 31 h 16 BrN 5 S: 569.0; test value: 569.4.
[0139]
[0140] In a 100ml three-necked flask, firstly mix S7 (7.5mmol), pinacol diborate (8.0mmol), (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com