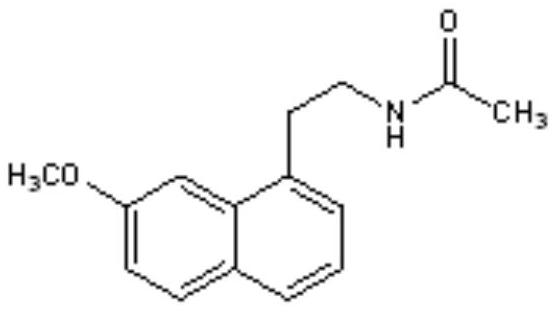
A kind of agomelatine transdermal patch and preparation method thereof
A transdermal patch and patch technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations containing active ingredients. It can solve adverse reactions, poor accuracy and uniformity of drug content, nano Porous silica is not easy to prepare and other problems, to achieve good penetration effect and increase the percutaneous penetration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 40g of agomelatine in 100mL of DMF, filter, and pour the filtrate into 3000mL of rapidly stirring distilled water at 25°C at room temperature. After adding, stir at room temperature, filter, wash the filter cake with distilled water, dry the product in vacuum at room temperature, and determine by HPLC method, the content of agomelatine crystals is greater than 99%. After grinding, sieve, the particle size of micronized Selected from 1μm-8μm.
[0036] The crystalline crystal uses Cu-Kα radiation, and the X-ray powder diffraction spectrum has characteristic diffraction peaks at 12.83, 13.86, 16.15, 18.57, 19.11, 20.87, 21.21 and 23.85 in terms of 2θ angle.
[0037] X-ray powder diffraction conditions:
[0038] Instrument: Rigaku D / MAX-2500 X-ray diffractometer; target: Cu-Kα radiation (λ=1.5405), 2θ=2-40°C; step angle: 0.04°C; calculation time: 0.5s; tube pressure: 40KV; Tube flow: 100mA; scanning speed: 8°C / min; filter: graphite monochromator; 2θ value error: ...
Embodiment 2
[0040] A preparation method of agomelatine transdermal patch, comprising the following preparation steps:
[0041] (1) Take by weighing the crystalline powder of the micronized agomelatine 100mg prepared in Example 1;
[0042] (2) Measure fucosterol, octanoic acid, 2-pyrrolidone and isopropanol at a volume ratio of 1:1:1:2, with a total volume of 5 mL. After mixing, ultrasonically mix for 15 minutes at an ultrasonic power of 150W; The powder of (1) was added to the mixture of fucosterol, octanoic acid, 2-pyrrolidone and isopropanol, and ultrasonically mixed for 45min under the ultrasonic power of 150W.
[0043] (3) Coating the dispersed microspheres on the polymer pressure-sensitive adhesive to obtain the patch; wherein, the hot-melt pressure-sensitive adhesive is heated to melt, and the dispersion prepared in the preceding steps is coated on the anti-aging Adhesive material PET film, dried at 70°C, and then compounded with the backing material flesh-colored double-elastic cl...
Embodiment 3
[0045]A preparation method of agomelatine transdermal patch, comprising the following preparation steps:
[0046] (1) Take by weighing the crystalline powder of the micronized agomelatine 100mg prepared in Example 1;
[0047] (2) Measure fucosterol, octanoic acid, 2-pyrrolidone and isopropanol at a volume ratio of 2:1:1:4, with a total volume of 10mL. After mixing, ultrasonically mix for 15min at an ultrasonic power of 150W; The powder of (1) was added into the mixture of fucosterol, octanoic acid, 2-pyrrolidone and isopropanol, and ultrasonically mixed for 60 minutes under the ultrasonic power of 150W.
[0048] (3) Coating the dispersed microspheres on the polymer pressure-sensitive adhesive to obtain the patch; wherein, the hot-melt pressure-sensitive adhesive is heated to melt, and the dispersion prepared in the preceding steps is coated on the anti-aging Adhesive material PET film, dried at 70°C, and then compounded with the backing material flesh-colored double-elastic c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com