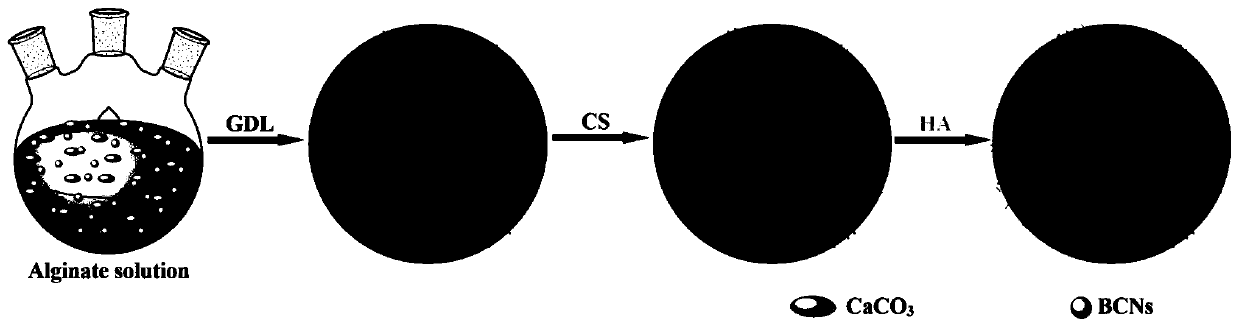
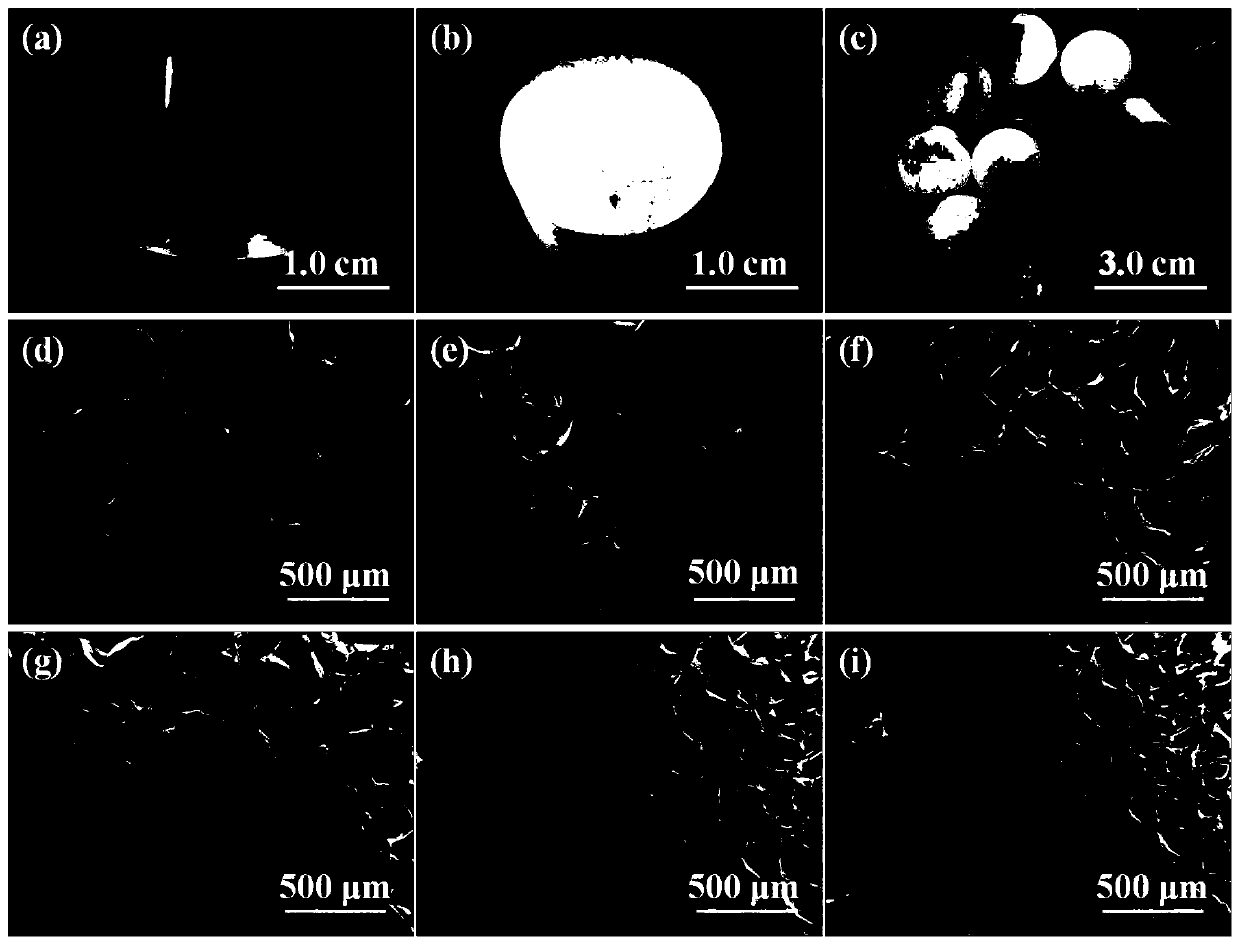
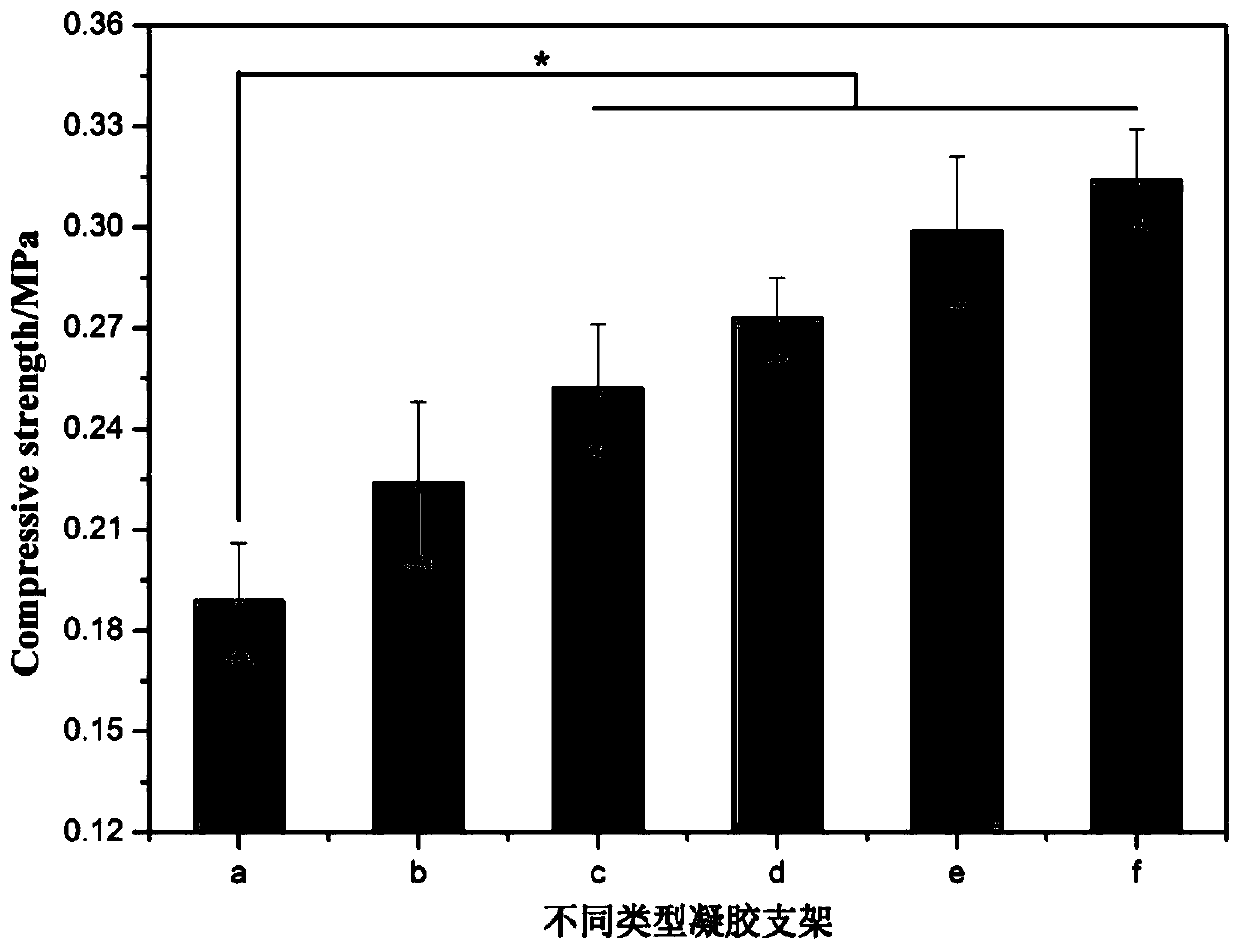
Functional drug-sustained-release medical dressing constructed by alginic acid aminated derivative/bacterial cellulose nanocrystal composite gel
A composite technology of bacterial cellulose and nanocrystals, used in medical science, bandages, etc., to achieve the effects of controlled release, improved mechanical strength, and uniform pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment B
[0051] The preparation of embodiment BCNs
[0052] Measure 100mL of naturally fermented coconut water, add 1.0g of sucrose, 0.5g of ammonium sulfate, 0.05g of magnesium sulfate and 0.15g of potassium dihydrogen phosphate to it, and mix thoroughly to make a fermentation medium. Before being inoculated with Acetobacter xylinum bacteria, the above-mentioned fermentation medium was sterilized at 120° C. for 30 minutes. Under the conditions of pH=4.0 and room temperature of 30° C., the initial bacterial cellulose gel was obtained by static culture for about 3 to 4 days. Then the bacterial cellulose gel was purified with 0.1mol / L NaOH aqueous solution at 80°C, and finally washed repeatedly with double distilled water until neutral. The obtained film was pulverized by a pulverizer, and then freeze-dried to obtain high-purity bacterial cellulose powder. Subsequently, 10 g of bacterial cellulose powder was dispersed in 100 mL of concentrated sulfuric acid solution with a mass fractio...
Embodiment 1
[0055] In this example, CaCO 3 / GDL complex is the cross-linking agent, and gentamicin is the antibiotic drug to be loaded. In the preparation of medical dressings, the CaCO 3 The molar ratio with GDL is 1:2, CaCO 3 The molar ratio of Ca element in SA to –COOH in SA is 0.36.
[0056] Dissolve 5 g of SA in 200 mL of distilled water, then add 50 mL of absolute ethanol and mix well. Then, 1.72 g (30% sodium alginate uronic acid monomer molar weight) of sodium periodate was added to the above mixed solution, and electric stirring was performed at room temperature for 24 hours in the dark. Add 10 mL of ethylene glycol to the above reaction solution, and magnetically stir for 2 h in the dark to terminate the reaction. The solution after the reaction was terminated was precipitated with 5 g of sodium chloride and 800 mL of absolute ethanol. The resulting precipitate was then redissolved in 100 mL of distilled water, and the solution was precipitated with 3 g of sodium chloride an...
Embodiment 2
[0060] In this example, CaCO 3 / GDL complex is the cross-linking agent, and ofloxacin is the antibiotic drug to be loaded, and in the preparation of medical dressings, the CaCO 3 The molar ratio with GDL is 1:2, CaCO 3 The molar ratio of Ca element in SA to –COOH in SA is 0.18.
[0061] Dissolve 5 g of SA in 200 mL of distilled water, then add 50 mL of absolute ethanol and mix well. Then, 1.72 g of sodium periodate was added to the above mixed solution, and electric stirring was carried out at room temperature for 24 h in the dark. Add 10 mL of ethylene glycol to the above reaction solution, and magnetically stir for 2 h in the dark to terminate the reaction. The solution after the reaction was terminated was precipitated with 5 g of sodium chloride and 800 mL of absolute ethanol. The resulting precipitate was then redissolved in 100 mL of distilled water, and the solution was precipitated with 3 g of sodium chloride and 600 mL of absolute ethanol. After repeating the pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| antibacterial rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com