Perfluoro-butyl substituted compound as well as preparation method and application thereof
A perfluorobutyl compound technology, which is applied in the field of perfluorobutyl substituted compounds and its preparation, can solve the problems of solubility, low stability, complex reaction steps, toxic by-products, etc., and eliminate the hazards of heavy metal catalysts , good air stability, beneficial to the effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
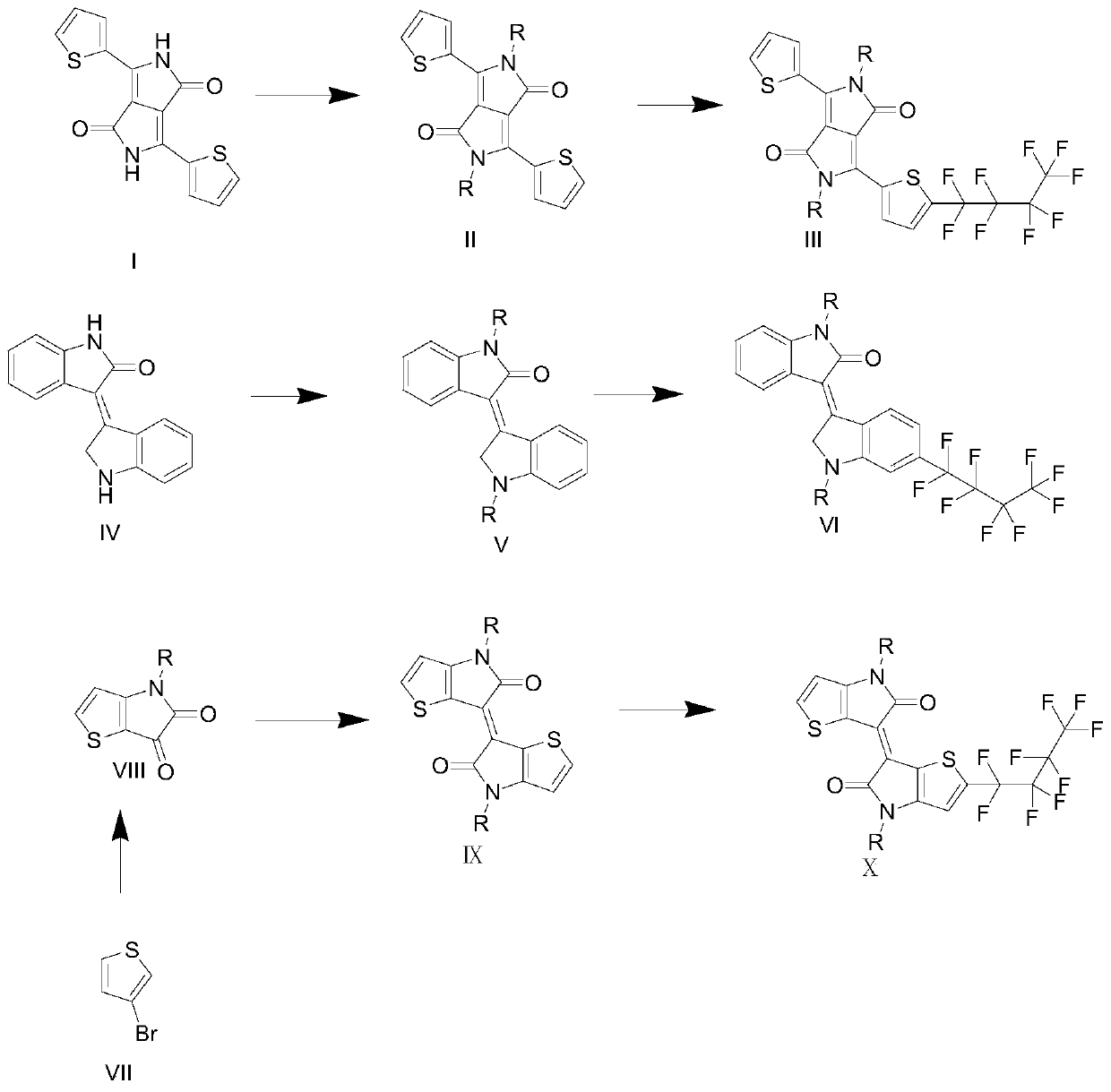
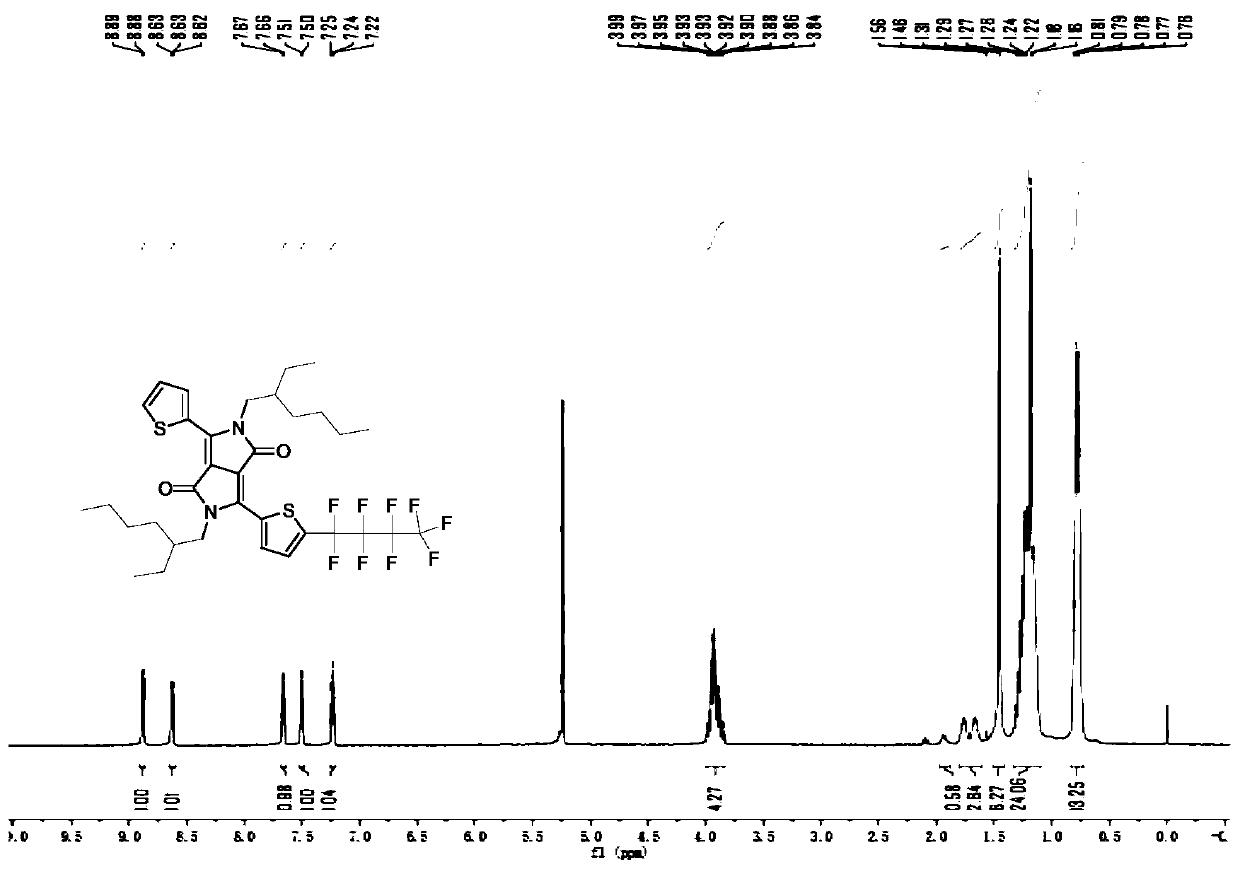
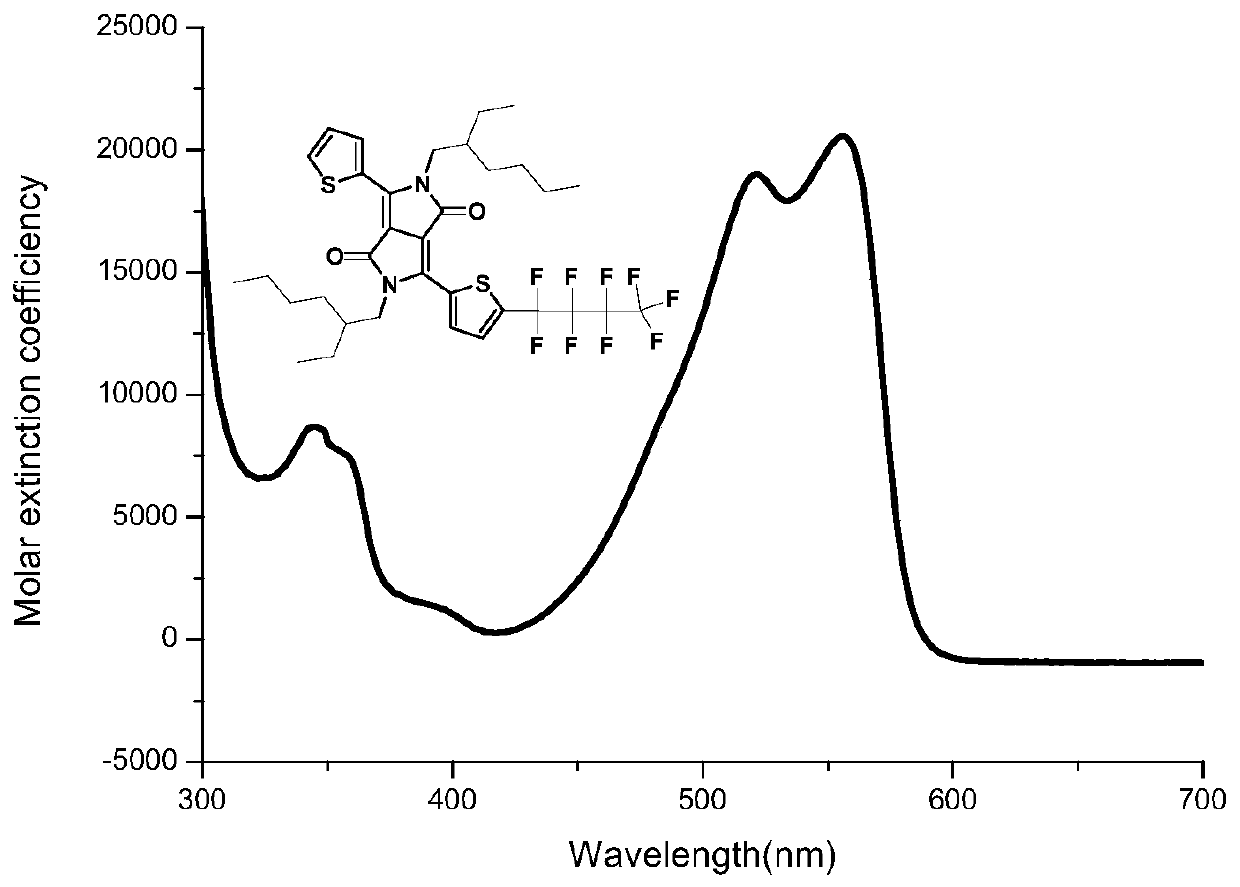
[0104] A preparation method of a perfluorobutyl substituted pyrrolopyrrole diketopyrrole, comprising the steps of:
[0105] (1) Add 29g of potassium tert-butoxide into a three-necked round-bottomed flask, start degassing and then fill with nitrogen for three consecutive times, then add 179mL of tert-amyl alcohol, and stir at 110°C for 2h. Then 20mL of 3-cyanothiophene was added dropwise and stirred for 45min, and 9.4mL of dimethyl succinate and 29mL of tert-amyl alcohol solution were added dropwise with a dropping funnel, stirred, and continued to stir for 3h. After the reaction solution was cooled to 80°C, 108 mL of methanol and 29 mL of deionized water were added and stirred for 45 min. After the solution was cooled to room temperature, 57 mL of hydrochloric acid and 287 mL of methanol were added and stirred for 45 min. The treated reaction solution was filtered, and the filter residue was washed with 72mL of methanol to obtain compound (I). The quality of the obtained compo...
Embodiment 2
[0113] A kind of preparation method of the perfluorobutyl substituent of isoindigo, comprises the steps:
[0114] (1) Add 3.7754g isatin, 3.4472g 2-indolinone and 1mL hydrochloric acid into a round bottom flask, then add 150mL acetic acid, stir, and react at 115°C for 12h; filter the reacted solution and wash with methanol, water, Sodium bicarbonate solution and pentane wash filter residue, obtain solid compound (IV), the quality of compound is 4.8537g, productive rate is 79%; Its chemical reaction equation is as follows:
[0115]
[0116] (2) 1.5g of compound (IV) and 7.905g of potassium carbonate were added to the round-bottomed flask, degassed and then filled with nitrogen for three consecutive times, then added 30mLN,N-dimethylformamide (DMF), stirred, and then Add 2.76g bromoisooctane and react at 100°C for 24h. The obtained solution was distilled under reduced pressure first, and then purified by a chromatographic column. The ratio of the eluent dichloromethane to pe...
Embodiment 3
[0122] A preparation method of a perfluorobutyl substituted substance of thiophene isoindigo, comprising the steps of:
[0123] (1) Add 0.2g of cuprous iodide and 5.6g of potassium carbonate into a round bottom flask, start to degas and then fill with nitrogen for three consecutive times, then add 10mL of N,N-dimethylformamide (DMF), stir, and then Add 2mL of 3-bromothiophene, 5mL of isooctylamine, and 0.74g of 2-isobutyrylcyclohexanone, and react at 120°C for 12h. The resulting solution is first distilled under reduced pressure, and then passed through a flash chromatography column. The eluent is dichloromethane , to obtain 3-secondary aminothiophene; 0.75mL oxalyl chloride was added to a round bottom flask, 25mL dichloromethane was added, and 1.9939g 3-secondary aminothiophene and 11mL dichloromethane solution were mixed with 5.71mL triethylamine and 40mL of dichloromethane solution was added dropwise in the round-bottomed flask successively, stirred, and reacted at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com