Tetraphenylethylene derivative fluorescent probe and preparation method thereof
A fluorescent probe, tetraphenylethylene technology, which is applied in the field of aggregation-induced luminescence fluorescent materials, can solve the problems of difficult preparation conditions of tetraphenylethylene derivatives, insoluble in water, etc., and achieves good water solubility, simple synthesis and wide application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of tetraphenylethylene derivative fluorescent probe
[0039] 1. Compound M 1 Synthesis
[0040] Take a clean 500mL three-necked flask, evacuate it several times, and add 4-methylbenzophenone (19.6g, 0.1mol) and zinc powder (20g, 0.308mol) to the three-necked flask under the protection of argon. Add 300mL tetrahydrofuran into the flask, turn on the magnetic stirrer to stir, seal a flask mouth with plastic wrap, and tie it tightly with a rubber band. Slowly pour liquid nitrogen into the ethanol bath to cool, when cooled to -78°C, measure 20mL of titanium tetrachloride 4, inhaled into the syringe, pierced the plastic wrap and slowly and evenly added to the flask, while continuously pouring liquid nitrogen to maintain the reaction temperature. After the feeding is completed, put a balloon on the spherical condenser, stir and react for 1.5 hours, and deflate the balloon in time. Stir the reaction for 1.5h, then change the oil bath and heat to ...
Embodiment 2
[0054] Example 2: M 3 Encapsulating doxorubicin as a drug carrier
[0055] Doxorubicin (DOX) was first dissolved in M 3 (246mg, 0.5mmol) in ethanol solution (25mL) (3.3×10 -3 M), add an aqueous solution (5mL) of zinc nitrate hexahydrate (150mg, 0.5mmol) at room temperature, and stir vigorously at room temperature to obtain DOX / Zn (M 3 )ball. The resulting coated metal-organic system was purified by centrifugation and washed multiple times with ethanol to give a red solid. The coated particles were redispersed in ethanol or phosphate buffer to obtain the corresponding colloids.
Embodiment 3
[0056] Embodiment 3: UV absorption spectrometry
[0057] (1) Target product M 3 Molecular UV Absorption Spectrometry
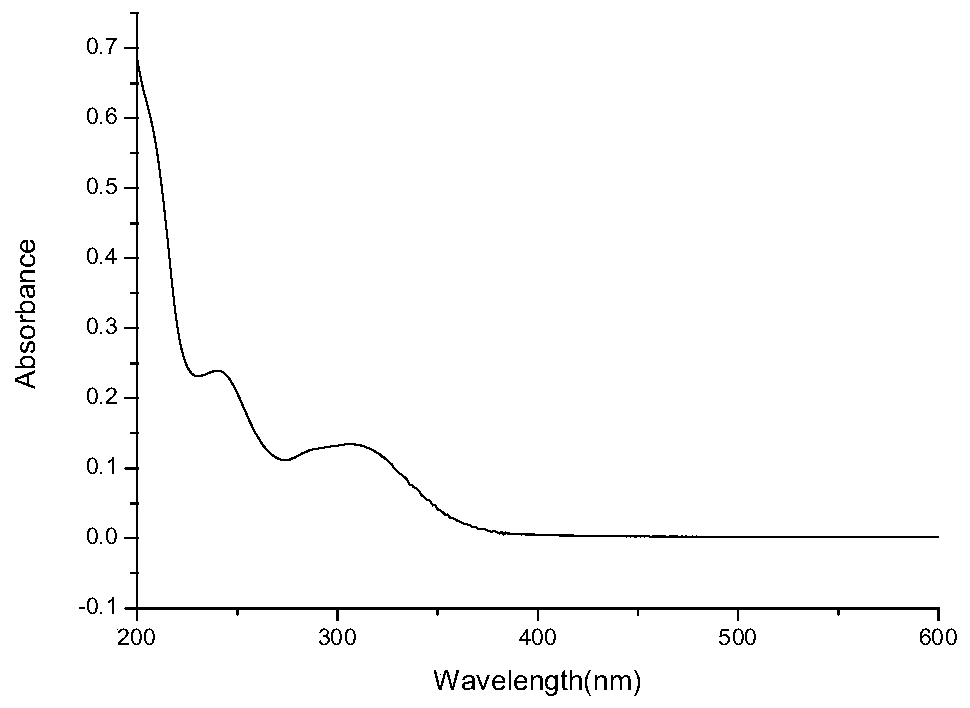
[0058] Pipet 2 mL of M 3 The molecule to be tested (0.01 mmol / L) was transferred to a 4 mL cuvette. The preset ultraviolet absorption wavelength range is determined between 200nm and 600nm, and a blank test is performed first to deduct the blank interference of the solvent used, and finally the ultraviolet absorption spectrum is measured. get figure 1 , which is M 3 The UV absorption spectrum of the molecule.
[0059] (2) Target product M 4 Molecular UV Absorption Spectrometry
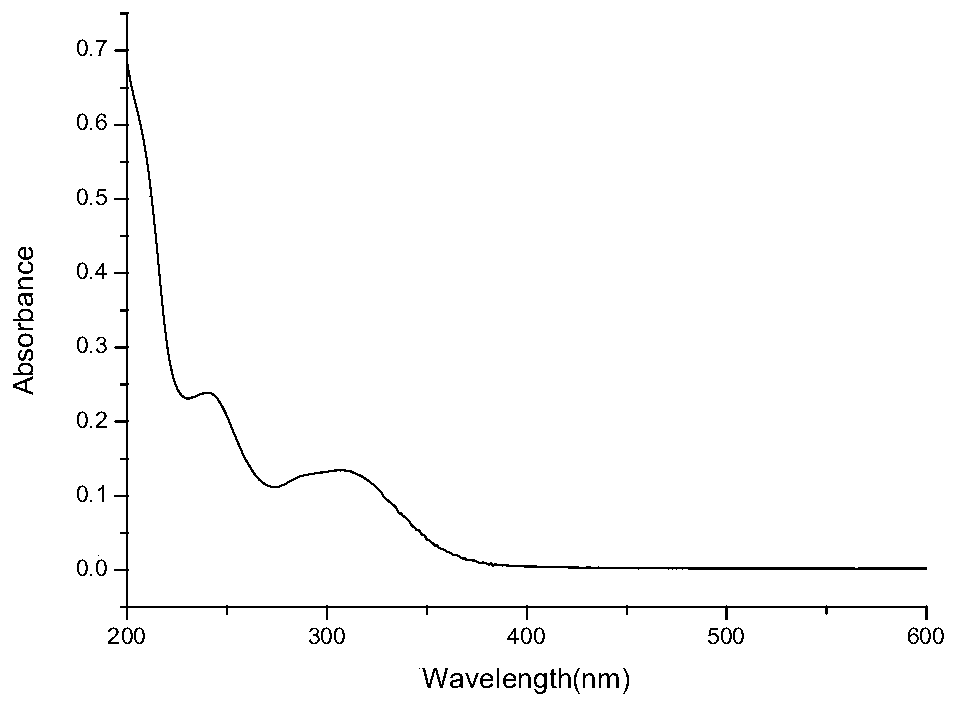
[0060] Pipet 2 mL of M 3 The molecule to be tested (0.05 mg / mL) was transferred to a 4 mL cuvette. The preset ultraviolet absorption wavelength range is determined between 200nm and 600nm, and a blank test is performed first to deduct the blank interference of the solvent used, and finally the ultraviolet absorption spectrum is measured. get figure 2 , which is M 4 The UV ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com