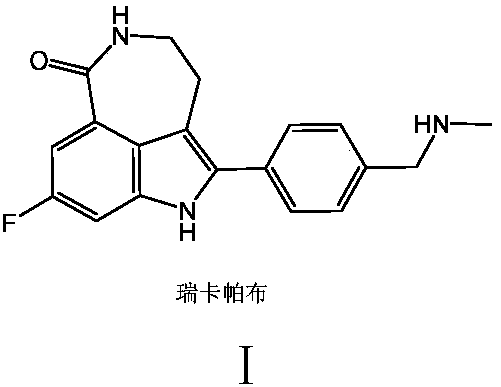
Simple and convenient production method of rucaparib
A technology for recapab and compounds, which is applied in the field of simple preparation of recapab, can solve the problems of long reaction process, uncontrolled temperature, unfavorable environmental protection and the like, and achieves the effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
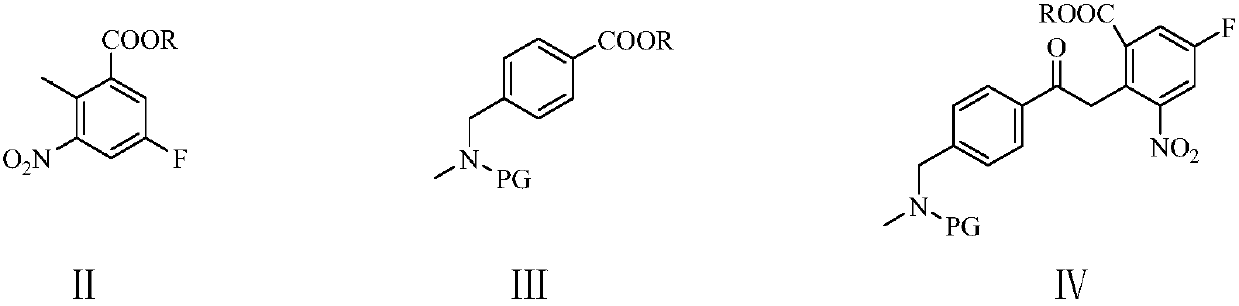
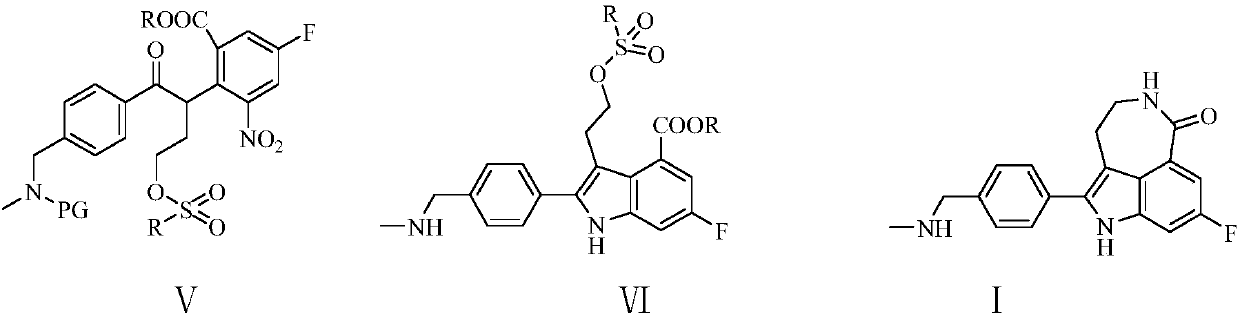
[0067] Example 1: 2-[1-(4-N-methyl-N-benzylaminomethyl)benzoyl-3-p-toluenesulfonate]n-propyl-3-nitro-5 Preparation of -methyl fluorobenzoate (Ⅴ1)
[0068] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 200 gram toluene, 27.0 gram (0.1 mole) 4-(N-methyl-N-benzylaminomethyl) methyl benzoate ( Ⅲ1), 13.5 grams (0.12 moles) of potassium tert-butoxide, heated to 50-55 ° C, dropwise added 22.0 (0.1 moles) 2-methyl-3-nitro-5-fluorobenzoic acid methyl ester (Ⅱ) And 50 grams of toluene mixture, dropwise, 50 ~ 60 ° C stirring reaction for 4 hours. Cool to 0-10°C, pass through 5.0 g of ethylene oxide, and react with stirring at 20-25°C for 4 hours. Add 12 grams of triethylamine, 21.0 (0.11 moles) of p-toluenesulfonyl chloride, and stir for 5 hours at 20-25°C. Add the reaction liquid to 250 g of ice water, adjust the pH value to 6-7 with saturated ammonium chloride solution, separate the layers, and extract the water layer twi...
Embodiment 2
[0069] Example 2: 2-[1-(4-N-methyl-N-benzylaminomethyl)benzoyl-3-benzenesulfonate]n-propyl-3-nitro-5-fluorobenzene Preparation of ethyl formate (Ⅴ1)
[0070] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser, add 200 gram tetrahydrofuran, 28.5 gram (0.1 moles) 4-(N-methyl-N-benzylaminomethyl) ethyl benzoate ( Ⅲ1), 13.5 grams (0.12 moles) of potassium tert-butoxide, heated to 50-55 ° C, dropwise added 23.0 (0.1 moles) ethyl 2-methyl-3-nitro-5-fluorobenzoate (Ⅱ) under stirring And 50 grams of tetrahydrofuran mixture, dropwise, 55 ~ 60 ° C stirring reaction for 4 hours. Cool to 0-10°C, pass through 5.0 g of ethylene oxide, and react with stirring at 20-25°C for 4 hours. Add 12 g of triethylamine and 19.5 g (0.11 mole) of benzenesulfonyl chloride, and stir and react at 20-25° C. for 5 hours. Add the reaction liquid to 200 g of ice water, adjust the pH value to 6-7 with saturated ammonium chloride solution, separate the layers, ...
Embodiment 3
[0071] Example 3: 2-[1-(4-N-methyl-N-benzylaminomethyl)benzoyl-3-methanesulfonate]n-propyl-3-nitro-5-fluorobenzene Preparation of ethyl formate (Ⅴ1)
[0072] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 200 g of 2-methyltetrahydrofuran, 28.5 g (0.1 mol) of 4-(N-methyl-N-benzylaminomethyl)benzene Ethyl formate, 8.6 grams (0.13 moles) of sodium ethylate, heated to 60-65 ° C, dropwise added 23.0 grams (0.1 moles) of ethyl 2-methyl-3-nitro-5-fluorobenzoate and 50 grams of 2 -Methyl tetrahydrofuran mixture, after dropping, stir and react at 65-70°C for 2 hours. Cool to 0-10°C, pass through 5.5 g of ethylene oxide, and react with stirring at 20-25°C for 4 hours. Add 12 grams of triethylamine and 15.0 (0.13 moles) of methanesulfonyl chloride, and stir and react at 20-25°C for 4 hours. Add the reaction liquid to 200 g of ice water, adjust the pH value to 6-7 with saturated ammonium chloride solution, separate the layers, and extrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com