Method for preparing iron/copper aza graphene zinc air battery cathode catalyst
A zinc-air battery and cathode catalyst technology, which is applied in the field of electrocatalytic materials and achieves the effects of simple preparation method, reduced time and energy consumption, and easy availability of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
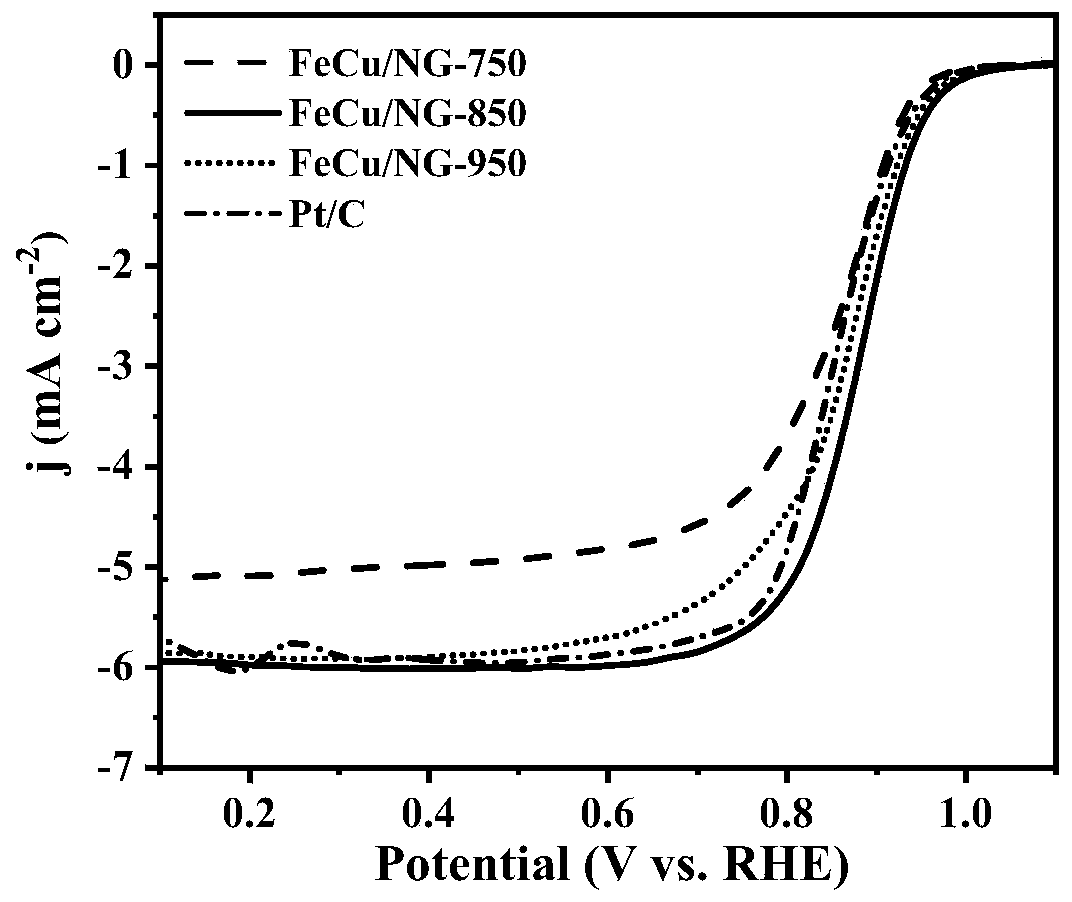
Embodiment 1
[0030] (1) Dissolve 1.1g of ferric chloride hexahydrate and 0.24g of sodium hydroxide in 20mL of deionized water, ultrasonically disperse evenly, transfer the mixed solution to a reaction kettle for hydrothermal reaction at 100°C for 4h, and cool to room temperature Afterwards, washing and drying obtains iron oxyhydroxide (FeOOH) precursor;
[0031] (2) Dissolve 0.23g of copper chloride dihydrate in 250mL of deionized water, add 200mg of polyethylene glycol, stir until dissolved, add 1.2mL of sodium hydroxide solution, stir at room temperature for half an hour, wash and dry , to get copper hydroxide (Cu(OH) 2 )Precursor;
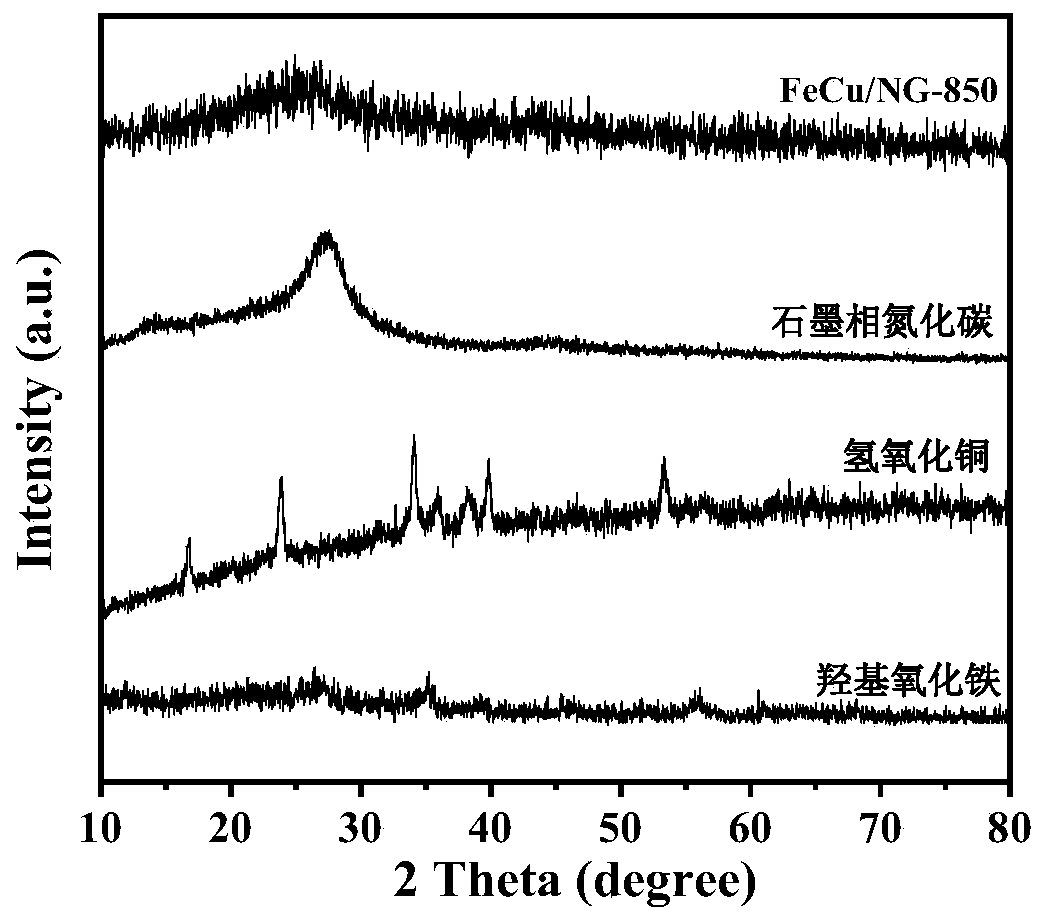
[0032] (3) Put 4g of urea in a tube furnace and calcinate at 600°C for 6h under an argon atmosphere to obtain graphite phase carbon nitride g-C 3 N 4 ;
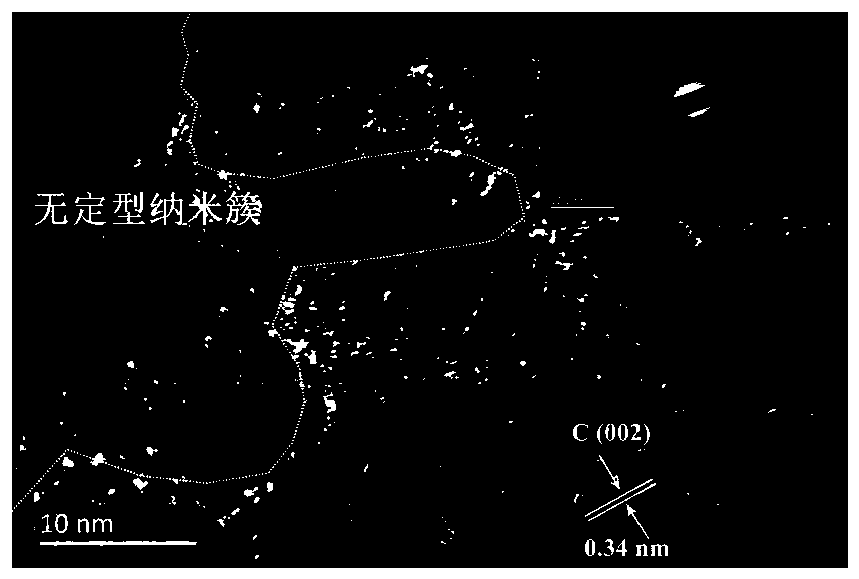
[0033] (4) Weigh 89mg of FeOOH and 97mg of Cu(OH) respectively 2 Dissolve in 10mL of deionized water, add it into 10mL of graphene oxide aqueous dispersion (2mg / mL) after ultrasonication for 30min, and...
Embodiment 2
[0037] (1) Dissolve 1.1g of ferric chloride hexahydrate and 0.24g of sodium hydroxide in 20mL of deionized water, ultrasonically disperse evenly, transfer the mixed solution to a reaction kettle for hydrothermal reaction at 100°C for 4h, and cool to room temperature Afterwards, washing and drying obtains iron oxyhydroxide (FeOOH) precursor;
[0038] (2) Dissolve 0.23g of copper chloride dihydrate in 250mL of deionized water, add 200mg of polyethylene glycol, stir until dissolved, add 1.2mL of sodium hydroxide solution, stir at room temperature for half an hour, wash and dry , to get copper hydroxide (Cu(OH) 2 )Precursor;
[0039] (3) Put 4g of urea in a tube furnace and calcinate at 600°C for 6h under an argon atmosphere to obtain graphite phase carbon nitride g-C 3 N 4 ;
[0040] (4) Weigh 89mg of FeOOH and 97mg of Cu(OH) respectively 2 Dissolve in 10mL of deionized water, add it into 10mL of graphene oxide aqueous dispersion (2mg / mL) after ultrasonication for 30min, and...
Embodiment 3
[0044] (1) Dissolve 1.1g of ferric chloride hexahydrate and 0.24g of sodium hydroxide in 20mL of deionized water, ultrasonically disperse evenly, transfer the mixed solution to a reaction kettle for hydrothermal reaction at 100°C for 4h, and cool to room temperature Afterwards, washing and drying obtains iron oxyhydroxide (FeOOH) precursor;
[0045] (2) Dissolve 0.23g of copper chloride dihydrate in 250mL of deionized water, add 200mg of polyethylene glycol, stir until dissolved, add 1.2mL of sodium hydroxide solution, stir at room temperature for half an hour, wash and dry , to get copper hydroxide (Cu(OH) 2 )Precursor;
[0046] (3) Put 4g of urea in a tube furnace and calcinate at 600°C for 6h under an argon atmosphere to obtain graphite phase carbon nitride g-C 3 N 4 ;
[0047] (4) Weigh 89mg of FeOOH and 97mg of Cu(OH) respectively 2 Dissolve in 10mL of deionized water, add it into 10mL of graphene oxide aqueous dispersion (2mg / mL) after ultrasonication for 30min, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com