Naphthalene sulfonamide compound and preparation methods and applications thereof
A technology of naphthalene sulfonamide and compound, applied in the field of medicinal chemistry, can solve the problems of high molecular polarity, reduced molecular polar surface area, unfavorable membrane penetration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
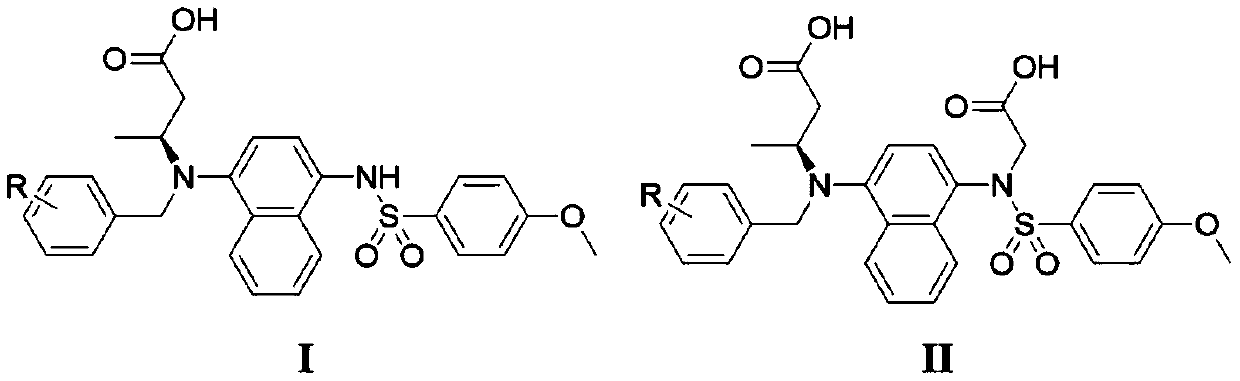
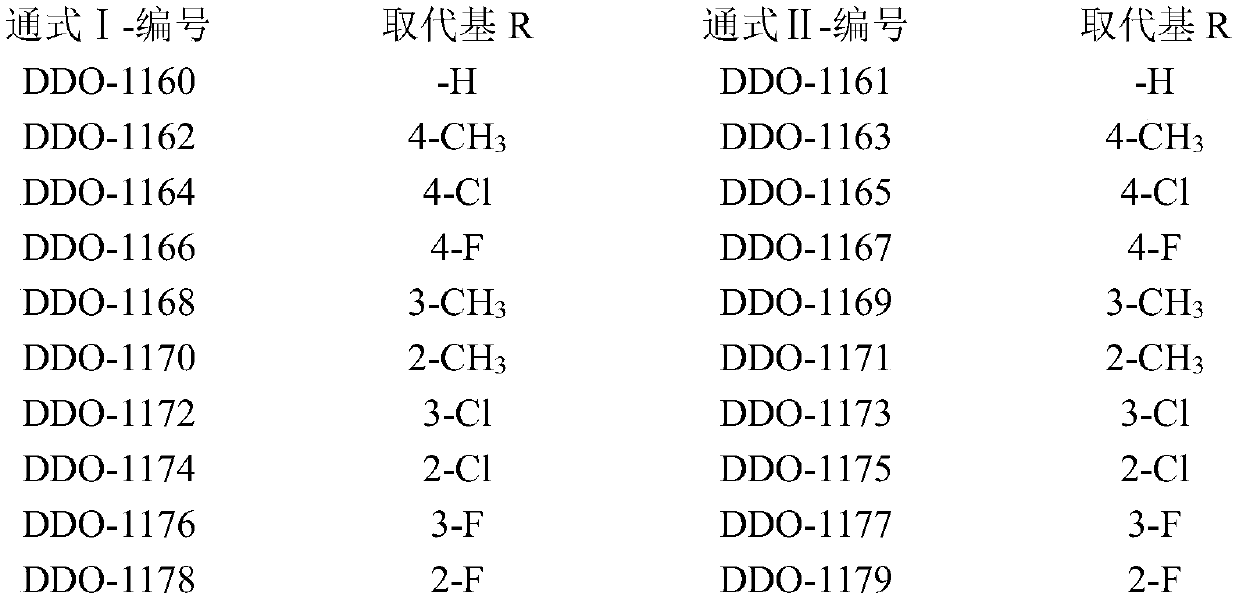
[0047] Example 1: Synthesis and structure confirmation of compounds
[0048] 1. General rules of experiment
[0049] All the chemical reagents used in this paper were commercially pure or analytically pure. Melting points were determined using a M.P. 50 MeltingPoint System (thermometer uncalibrated). 1 H-NMR, 13 C-NMR nuclear magnetic resonance spectrum is measured by Bruker AV300 type (300MHz) nuclear magnetic resonance instrument (TMS is an internal standard substance, mass spectrum is measured by Agilent 1946A-MSD type mass spectrometer (ESI-MS), Water Q-Tof type mass spectrometer (HRMS) , the purity was determined by HPLC, the chromatographic column was an Agilent C18 (4.6×150 mm, 3.5 μM) type reversed-phase column, and the mobile phase was methanol:water:trifluoroacetic acid=85:15:0.1.
[0050] The concentration of the solvent used was the N-1100 rotary evaporator produced by EYELA Instrument Co., Ltd. (carrying out at 40 ° C), the silica gel used for column chromatogr...
Embodiment 2
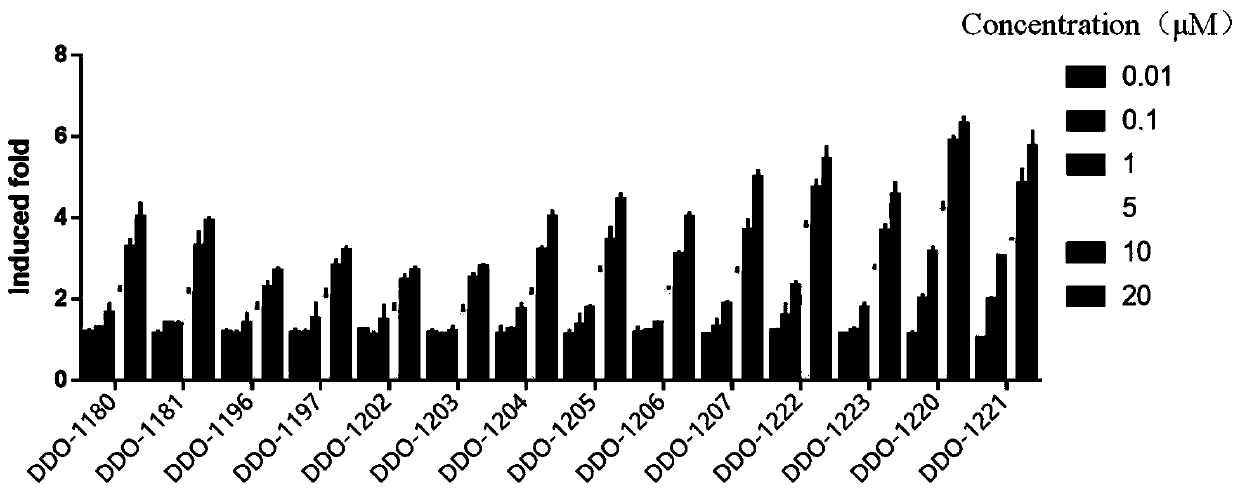
[0453] Embodiment 2: the active test of compound
[0454] 1. Keap1-Nrf2PPI competitive inhibition test based on fluorescence polarization (FP experiment)
[0455] For the FP experiment, a 384-well black plate produced by Corning (model 3676) was used, and the total reaction volume was 40 μL. 20 μL of compound, 10 μL of 4 nM FITC-labeled Nrf2 9 peptide, and 10 μL of 12 nM Keap1 Kelch domain protein were added to the wells in the order. For positive control, use 20 μL 200 nM DDO-1002, 10 μL 4 nM FITC-labeled Nrf2 9 peptide, 10 μL 12 nM Keap1 Kelch domain protein, for negative control, use 20 μL HEPES buffer, 10 μL 4 nM FITC-labeled Nrf2 9 peptide, 10 μL 12 nM For the Keap1 Kelch domain protein blank control, 10 μL of 4 nM FITC-labeled Nrf2 9 peptide and 30 μL of HEPES buffer were used. After addition, incubate at room temperature for 30 min. The detection instrument is SpectraMax Multi-Mode Microplate Reader (Molecular Devices), the excitation light wavelength is 485nm, the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com