Lysosome-targeted photo-controlled fluorescent molecular switch, and synthesis method and application thereof
A technology of fluorescent molecules and synthesis methods, applied in the field of light-controlled fluorescent molecular switches and their synthesis, can solve the problems of photoactivation performance failure, limited application, fluorescence interference, etc., and achieve good imaging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 3-amino-substituted rhodamine ethyl morpholine spiroamide (P1) synthetic route and product structure are as follows:
[0025]
[0026] 3-Nitrorhodamine (2 mmol, 0.974 g) and 2-ethylaminomorpholine (2 mmol, 0.146 g) were dissolved in absolute ethanol (35 mL). The temperature was raised to 78°C for reflux, and after stirring for 4 hours, the solvent was evaporated under reduced pressure, and the product was separated and purified by column chromatography (silica gel, petroleum ether / ethyl acetate, 4:1v / v) to obtain a light yellow powder (1.14g, 95 %). Then all the powder was dissolved in methanol (5mL), stirred under hydrogen atmosphere and palladium carbon (10%wt) catalysis for 1 hour, the filtrate was taken by suction filtration, and the final white powder product P1 (1.07g , 99%).
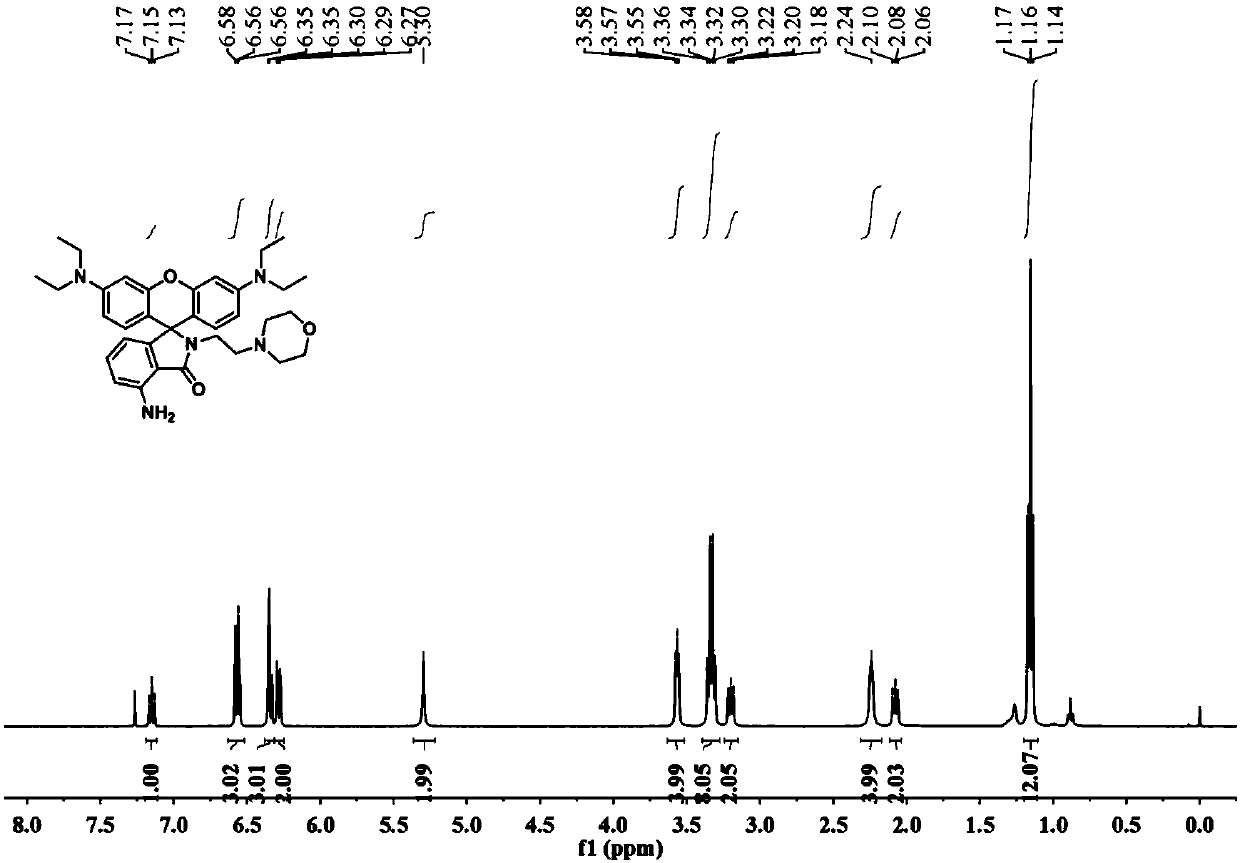
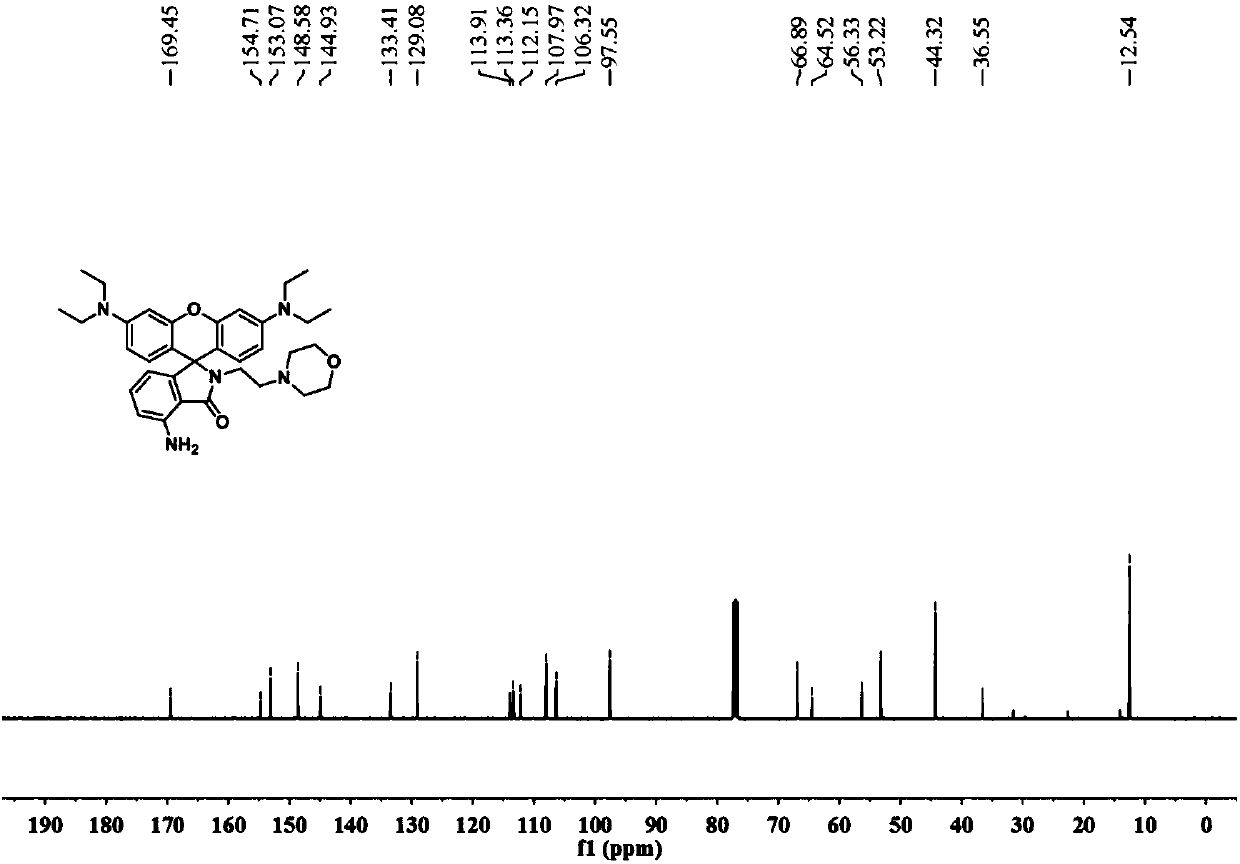
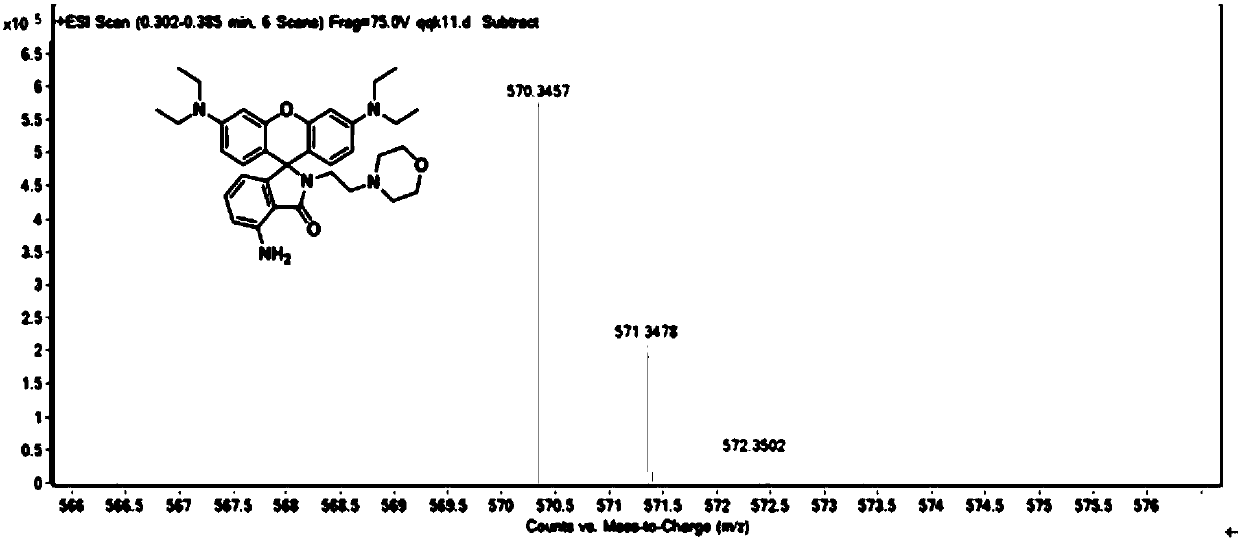
[0027] The powder product was characterized by NMR, mass spectrometry and high resolution mass spectrometry. figure 1 , figure 2 , image 3 shown.
[0028] 1 H NMR (400MHz, CDCl...
Embodiment 2
[0031] The product P1 in Example 1 was dissolved in methylene chloride / methanol (9 / 1, v / v) mixed solvent (concentration is 10 - 5 M), trifluoroacetic acid (2.3 μL, 1000 eq) was added to the mixed solution. Time-resolved UV-Vis absorption spectra were measured before and after acid addition ( Figure 4 ). The results showed that the absorbance at the maximum absorption wavelength of P1 did not increase with the increase of acidification time, indicating that the P1 molecule had the property of acid resistance.
Embodiment 3
[0033] MCF-7 cells were co-stained with the product 3-amino-substituted rhodamine ethylmorpholine spiroamide P1 (10 μM) in Example 1 and a commercial lysosomal labeling dye (LTG, 0.1 μM), and the MCF-7 cells were cultured by laser confocal An inverted microscope was used to observe the fluorescent staining in the two channels in real time. The excitation light wavelength of the green channel was 488nm, and the fluorescence signal in the 500-550nm band was collected. The excitation light wavelength of the red channel was 561nm, and the fluorescence signal in the 580-653nm band was collected. Comparative observation found that the fluorescent signal in the lysosome can be observed in the green channel after 0.5 hours of staining, while the red channel still has no obvious fluorescent signal in the lysosome after 2 hours of staining, and then irradiated in situ with 375nm ultraviolet light. The cells were irradiated, and the fluorescence images of the two channels were collected f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com