Polypeptide, anti-tumor drug made from polypeptide and preparation method of anti-tumor drug
A technology for tumor drugs and fat-soluble drugs, which is applied in the preparation of tumor drugs and in the field of tumor drugs. It can solve the problems of difficult assembly and preparation, easy to cause inflammation, poor biocompatibility, etc., and achieve low synthesis cost, convenient purification and stability. good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of Ac-IIIIIIKKKKK-NH2 polypeptide
[0055] Step 1. Distill N,N-dimethylformamide (DMF) and piperidine (Piperidine) solvents
[0056] Distill the purchased DMF solution under reduced pressure at 60°C to obtain pure DMF solvent; add a small amount of CaH2 to the purchased piperidine and heat to reflux for 1-2 hours, and receive the fraction with boiling point temperature (106°C) to obtain pure piperidine pyridine solvent.
[0057] Step 2, preparation of amino acid, resin, activator, capping agent, deprotecting agent
[0058] Calculate the amount of amino acids and other reagents required for the preparation of 0.25mM NH2-IIIIIIKKKKK-NH2 on the polypeptide solid-phase synthesizer:
[0059] Lys (lysine): 2.54g dissolved in 27mL DMF;
[0060] Ile (isoleucine): 2.27g dissolved in 32mL DMF;
[0061] Resin (loaded at 0.6mmol / g): 0.417g;
[0062] Activator: Diisopropylcarbodiimide (DIC): 17mL;
[0063] Activated base: 17-(Acetyloxy)-3-Methoxy-20-oxo...
Embodiment 2
[0069] Embodiment 2: Preparation of Ac-IIIIIIKKKKK-NH2 drug-loaded carrier
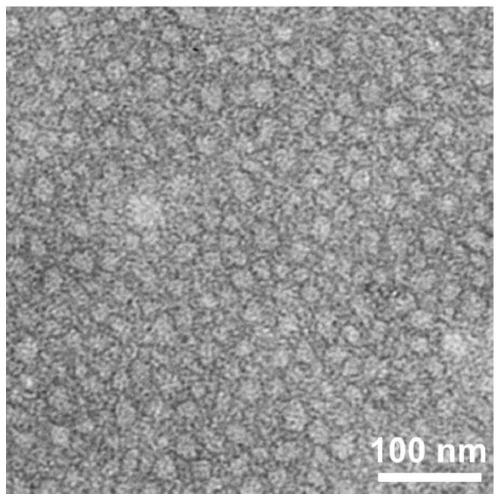
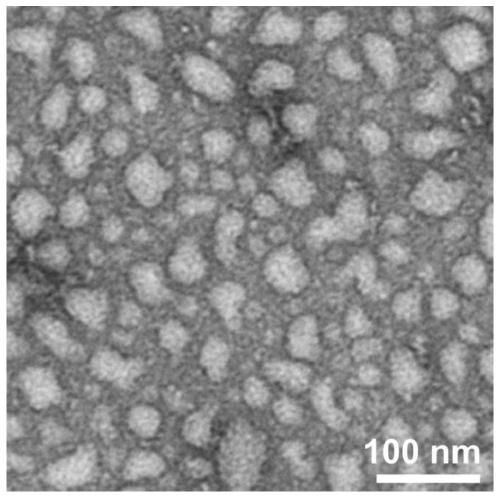
[0070] Weigh 0.519mg (0.5mmol / L) of peptide Ac-IIIIIIKKKKK-NH2, add 1mL of PBS buffer solution (pH7.4), sonicate for 5min, and after standing at room temperature for 4h, TEM observation results show that nanoparticle self-assembly has been formed at this time body (such as figure 1 shown).
Embodiment 3
[0071] Example 3: Detection of the self-assembly morphology of Ac-IIIIIIKKKKK-NH2 drug-loaded carrier in PBS buffer
[0072] The specific detection method is as follows:
[0073] Examination of self-assembled morphologies in PBS buffer (TEM).
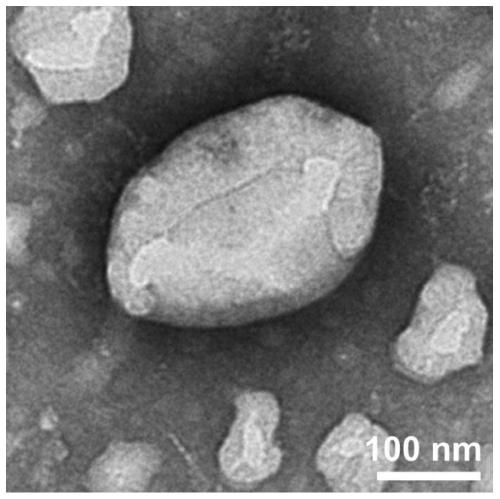
[0074] TEM: Adjust the pH of the peptide solution to 6.0, and after standing at room temperature for a certain period of time, pipette 50 μL of the peptide solution of the sample onto the parafilm, and then adsorb it on the pure carbon membrane for 3 minutes. Excess solution was removed with filter paper, and the surface was air dried. The sample was stained with a small amount of phosphotungstic acid dye (1.0%) for 3 minutes, and then the dye was removed with filter paper. The samples were air-dried and tested. HITACHIHT7700 instrument (Japan) was used for transmission electron microscopy (TEM) research. The results showed that the peptide sample Ac-IIIIIIKKKKK-NH2 observed by transmission electron microscopy self-assembled into a n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com