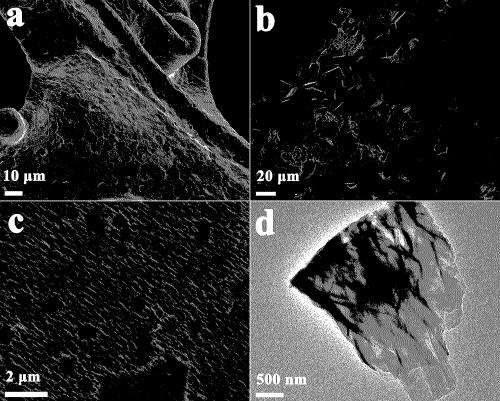
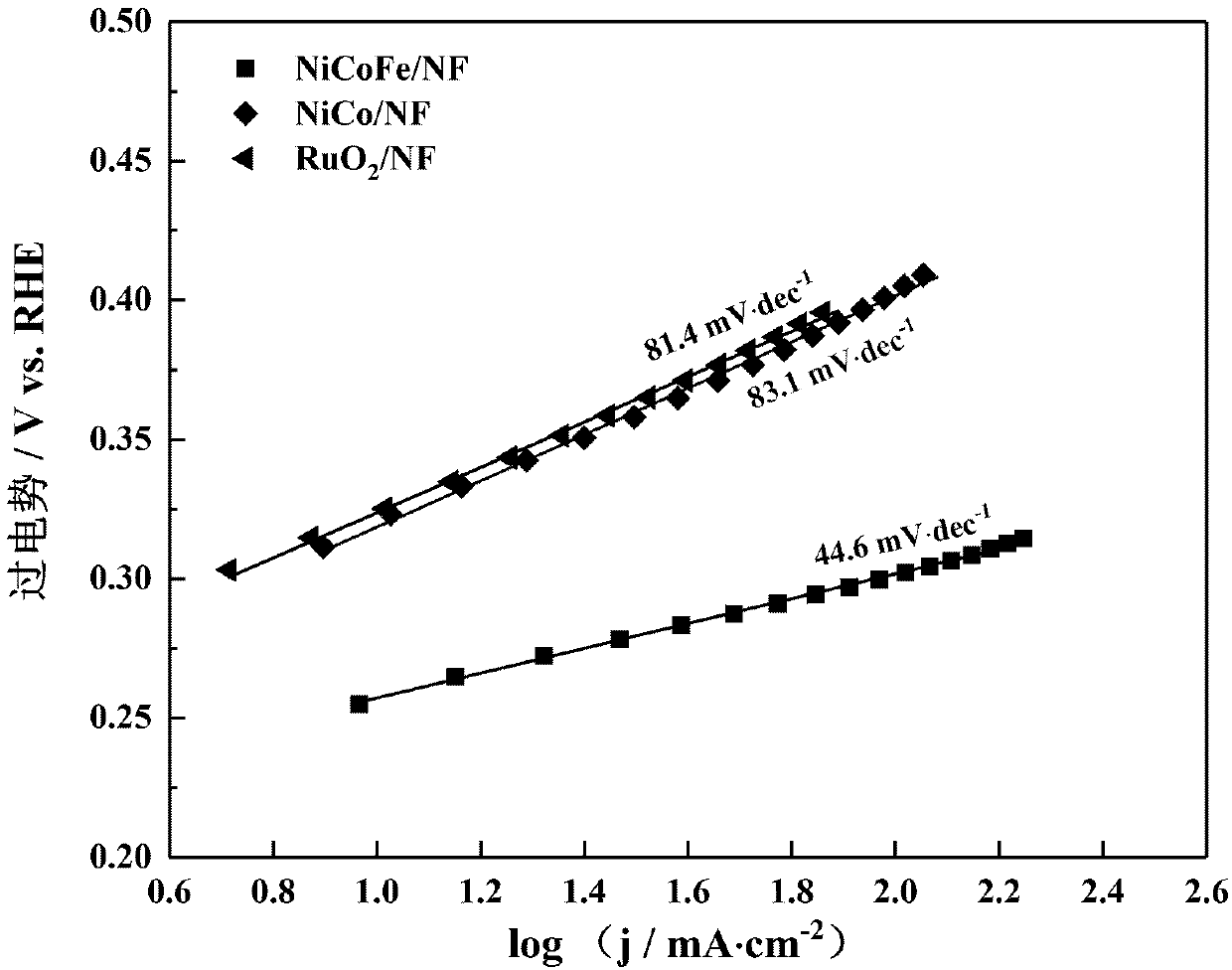
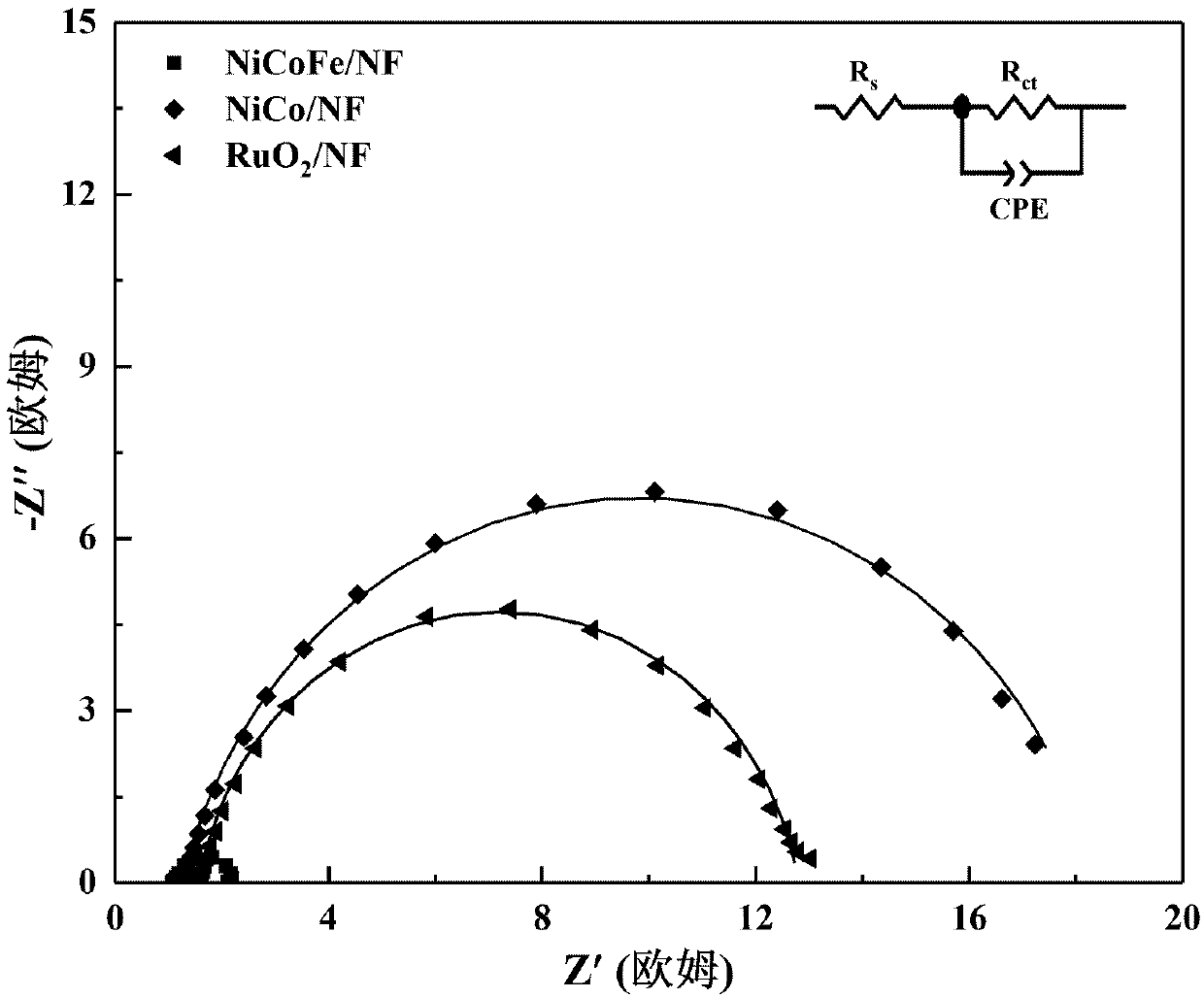
Preparation method of non-noble metal NiCoFe/NF electrocatalyst and application of electrocatalyst to oxygen evolution
An electrocatalyst and non-precious metal technology, applied in the field of electrocatalysis, can solve the problems of limiting large-scale commercial application, slow kinetics, and scarce reserves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 0.60mmol of nickel acetate, 3.0mmol of cobalt acetate and 10.0mmol of 2-methylimidazole into 40.0ml of anhydrous methanol, sonicate for 20 minutes to fully dissolve, then transfer to a 100ml reaction kettle and put Add the pretreated nickel foam, set the temperature at 130°C, and the reaction time is 15 hours. After the reaction is completed, let it cool naturally and rinse the obtained sample (NiCo / NF) with methanol and secondary water, and dry it naturally for later use. .
[0029] (2) Dissolve 0.75mmol of ferrous sulfate in 10.0ml of secondary water, stir to dissolve it completely, then immerse the NiCo / NF sample obtained in the above steps for 0.5 hours, and take out the obtained sample after the end (NiCoFe / NF NF-0.5h) and rinsed with secondary water, dried for testing.
Embodiment 2
[0031] (1) Dissolve 0.60mmol of nickel acetate, 3.0mmol of cobalt acetate and 10.0mmol of 2-methylimidazole into 40.0ml of anhydrous methanol, sonicate for 20 minutes to fully dissolve, then transfer to a 100ml reaction kettle and put Add the pretreated nickel foam, set the temperature at 130°C, and the reaction time is 15 hours. After the reaction is completed, let it cool naturally and rinse the obtained sample (NiCo / NF) with methanol and secondary water, and dry it naturally for later use. .
[0032] (2) Dissolve 0.75mmol of ferrous sulfate in 10.0ml of secondary water, stir to dissolve it completely, then immerse the NiCo / NF sample obtained in the above steps for 1.0 hour, and take out the obtained sample after the end (NiCoFe / NF NF-1.0h) and rinsed with secondary water, dried for testing.
Embodiment 3
[0034] (1) Dissolve 0.60mmol of nickel acetate, 3.0mmol of cobalt acetate and 10.0mmol of 2-methylimidazole into 40.0ml of anhydrous methanol, sonicate for 20 minutes to fully dissolve, then transfer to a 100ml reaction kettle and put Add the pretreated nickel foam, set the temperature at 130°C, and the reaction time is 15 hours. After the reaction is completed, let it cool naturally and rinse the obtained sample (NiCo / NF) with methanol and secondary water, and dry it naturally for later use. .
[0035] (2) Dissolve 0.75mmol of ferrous sulfate in 10.0ml of secondary water, stir to dissolve it completely, then immerse the NiCo / NF sample obtained in the above steps for 2.0 hours, and take out the obtained sample after the end (NiCoFe / NF NF-2.0h) and rinsed with secondary water, dried for testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com