Fluorine-containing benzophenone derivative and preparation method and application thereof
A technology of benzophenone and dihydroxybenzophenone, applied in the field of fluorine-containing benzophenone derivatives and its preparation, can solve problems such as easy yellowing of cured film, achieve inhibition of oxygen inhibition, enhance Good photostability and compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
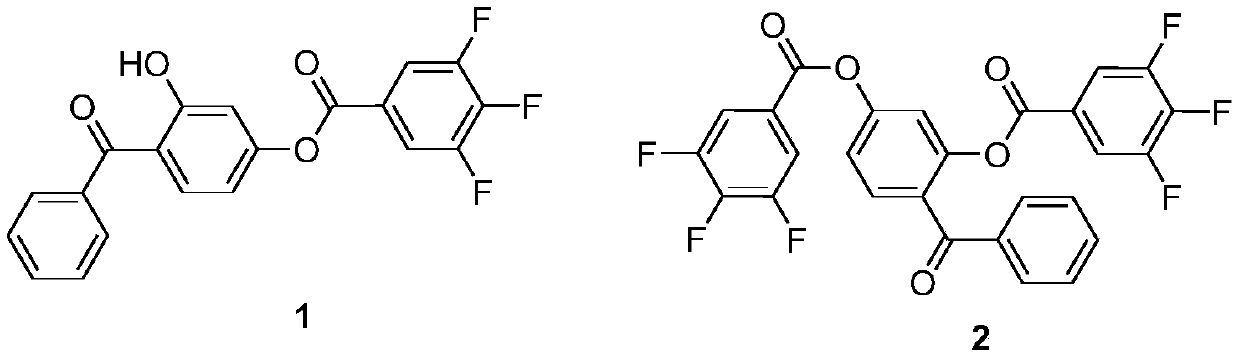
[0020] Add 0.214g (1mmol) of 2,4-dihydroxybenzophenone and 0.150g (1.5mmol) of triethylamine to the container, dissolve it with 3ml of dichloromethane, and slowly add 0.388g (2mmol) under ice-water bath conditions. ) of 3,4,5-trifluorobenzoyl chloride, stirred for 12 hours. After the reaction, add water to the reaction solution and extract with ethyl acetate, wash with water after collecting the organic phase, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation; and use petroleum ether and ethyl acetate (volume ratio 5:1) as The eluent is separated by a chromatographic silica gel column to obtain derivative 1 and derivative 2.
[0021] 2-Hydroxy-4-(3,4,5-trifluorobenzoyloxy)benzophenone (derivative 1): white solid, yield: 45%, m.p.25-27°C. 1 H NMR (600MHz, CDCl 3 )δ12.33(s,1H),7.88(t,J=7.0Hz,2H),7.75–7.69(m,3H),7.64(t,J=7.5Hz,1H),7.55(t,J=7.7 Hz,2H),6.97(d,J=2.3Hz,1H),6.78(dd,J=8.8,2.3Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ200.78,164.86,161...
Embodiment 2
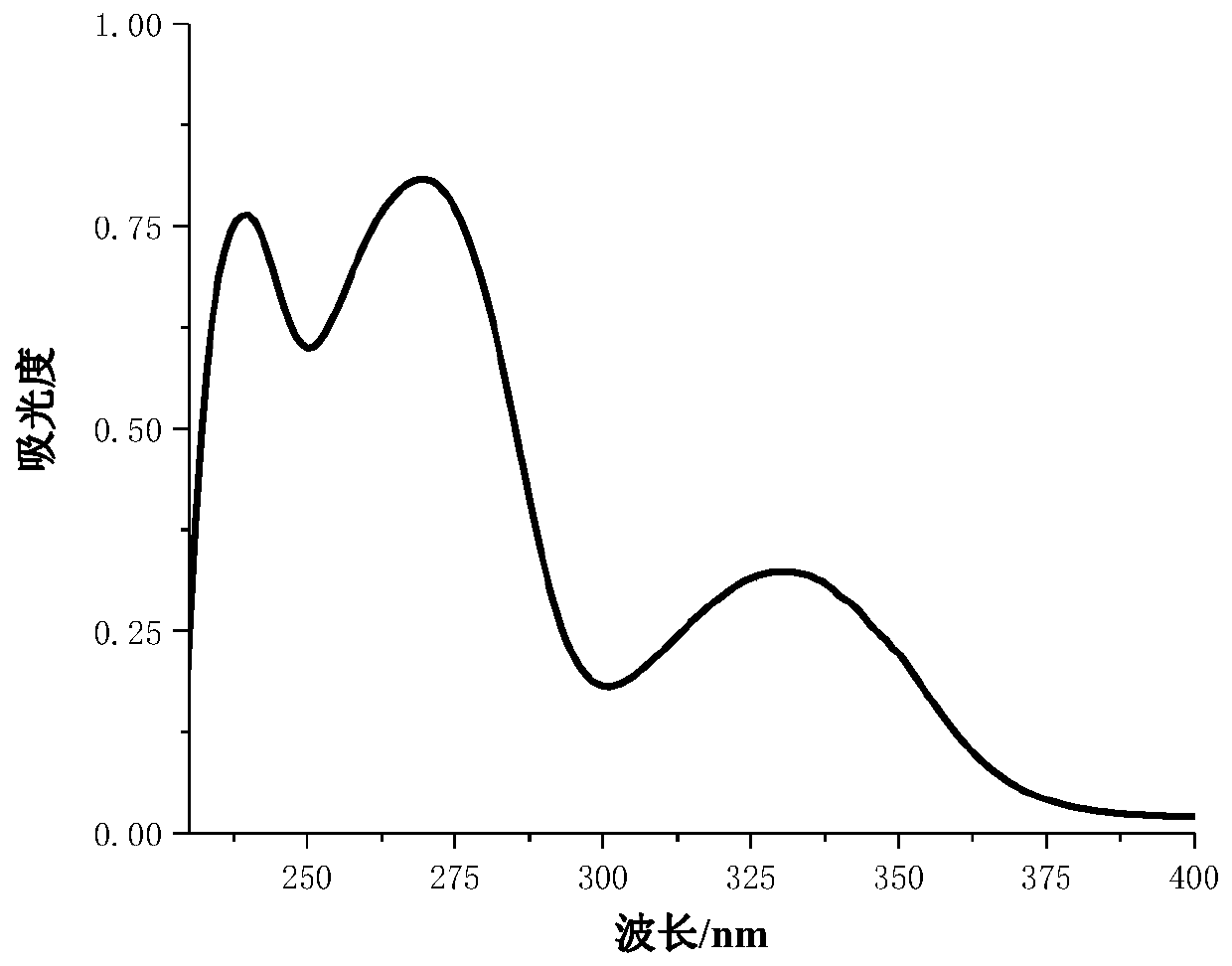
[0025] Embodiment 2: Derivative 1 is used in the performance evaluation of ultraviolet absorber
[0026] Experimental recipe:
[0027]
[0028] Working conditions:
[0029] Add epoxy propylene carboxylic acid resin 50g, 1,6-hexanediol diacrylate 44g, photoinitiator (1173) 3g, triethanolamine 2g, 2-hydroxyl-4-(3) in the glass container that stirrer is housed , 1g of 4,5-trifluorobenzoyloxy)benzophenone, stirred to make it dispersed evenly and transparently, and stood still for 5-10 minutes to obtain a transparent free radical photocurable coating. The paint is divided into three parts, one part is put into a transparent glass bottle and covered for daily indoor storage; the other part is put into a transparent glass bottle and covered with a cover for indoor dark storage; one part is coated on a glass plate with a brush, and the film The thickness is 75 μm, and then cured by a UV curing instrument at a speed of 5 meters per minute (about 5 seconds of light time).
Embodiment 3
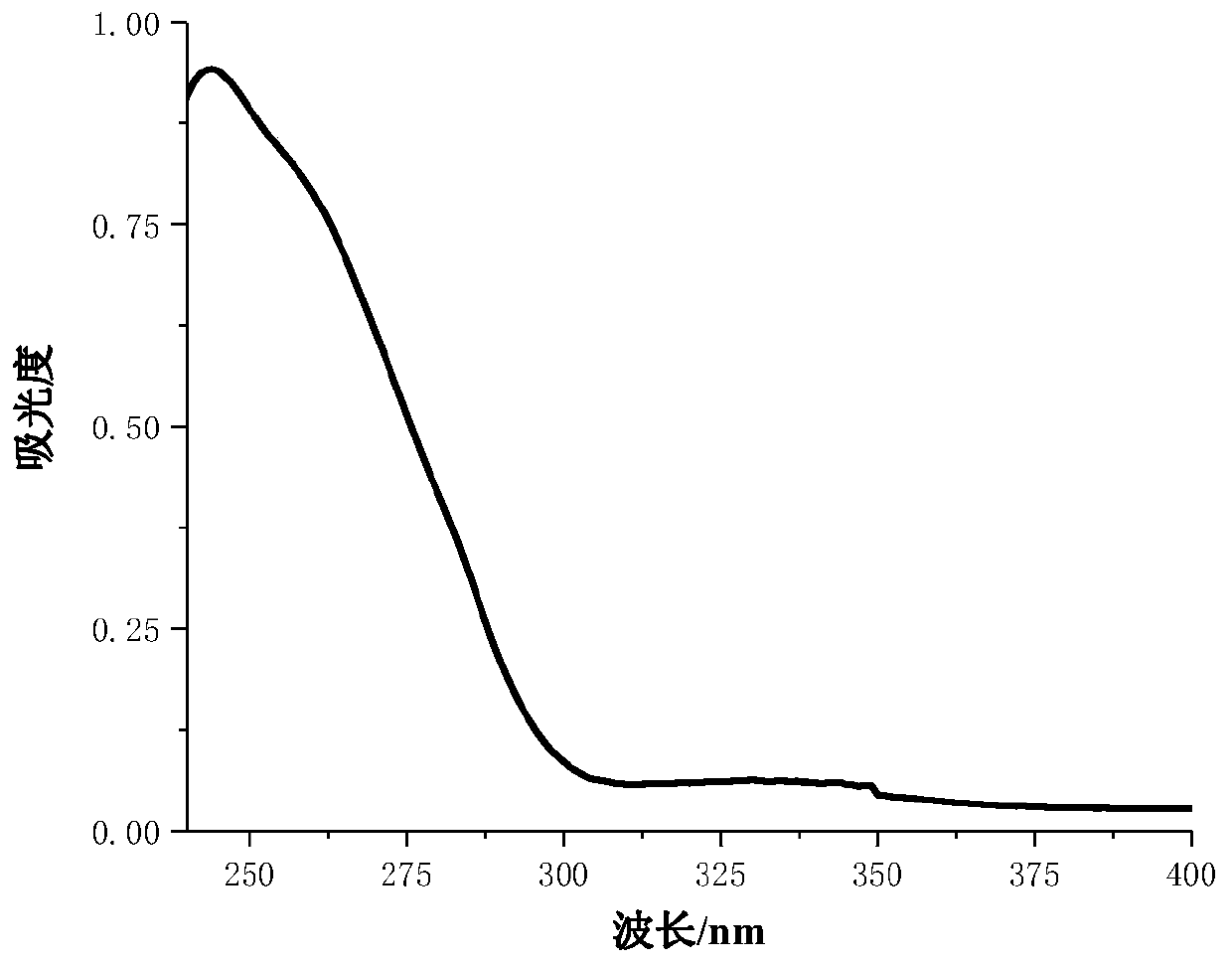
[0054] Embodiment 3: Applied performance evaluation of derivative 2
[0055] Experimental formula:
[0056]
[0057] working conditions
[0058] Under the condition of avoiding light, add 20.03g of 2,4-bis(3,4,5-trifluorobenzoyloxy)benzophenone and 0.5g of modified epoxy acrylate (UV1005-65) into the glass container , 0.45g of 1,6-hexanediol diacrylate (HDDA), 0.02g of triethanolamine, stir well until the coating liquid becomes transparent. The mixture was coated on a glass plate with an applicator to form a film with a film thickness of 75um. It was irradiated and cured in a medium-pressure mercury lamp, and the power of the mercury lamp was 400W.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com