Method for catalyzing hydrogen production of formic acid by iridium-immobilized metal organic framework material
A metal-organic framework, solid-supported technology, used in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., to avoid deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0038] 0.68g NH 2 Add BDC and 100mL DMF to a flask placed in an oil bath, stir magnetically until the solid is completely dissolved; after the temperature rises to 110°C, use a constant pressure funnel to dissolve the 3 ·6H 2 O solution (1.80g AlCl 3 ·6H 2 (0 and 50mL DMF mixed with ultrasound) were slowly added dropwise to the flask within 90min; after continuing to stir at a constant temperature for 3h, turn off the stirring and let stand for 20h; after the reaction was completed, slowly drop to room temperature and suction filter under reduced pressure, and wash the yellow solid three times with DMF. Then the obtained crude product was extracted overnight with ethanol Soxhlet, dried and activated under vacuum at 100°C to obtain pure NH 2 -MIL-101(Al).
[0039] 0.07g DMAP, 50mLDMA, 0.96g NH 2 -MIL-101(Al) was mixed evenly and added to the autoclave, and reacted at 80°C for 30 minutes in a nitrogen atmosphere; after cooling down, 0.75g PCCH was added under magnetic stirr...
Embodiment 2
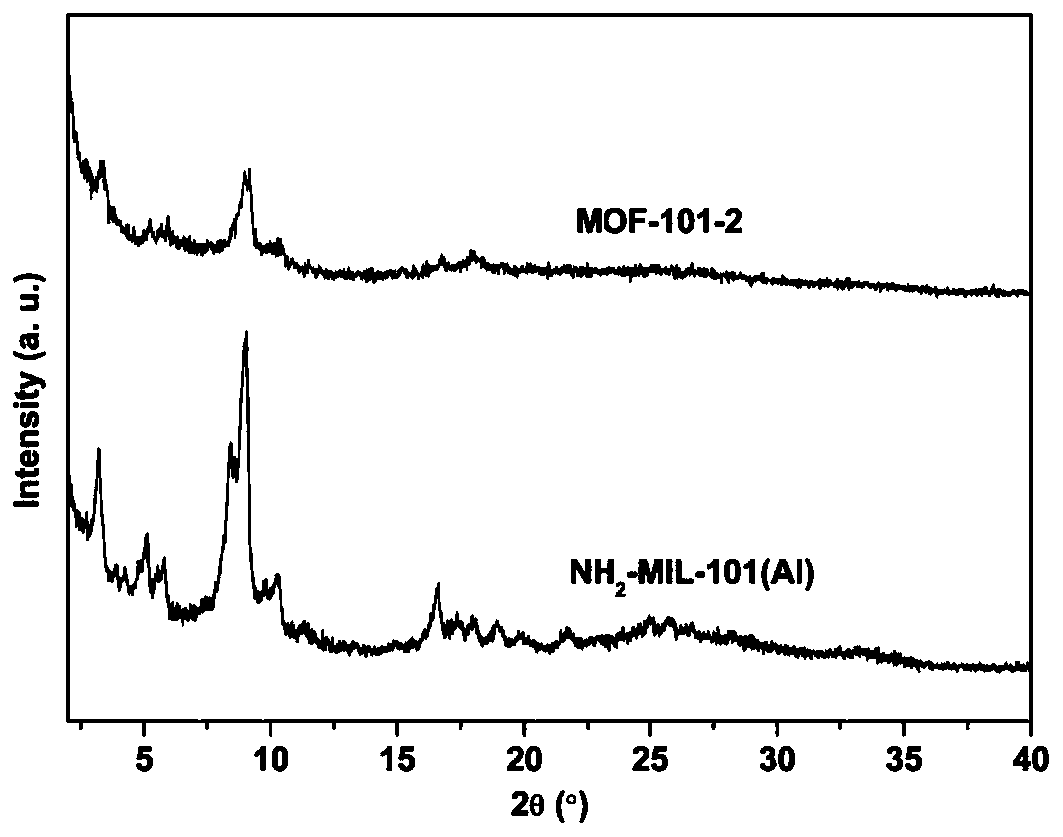
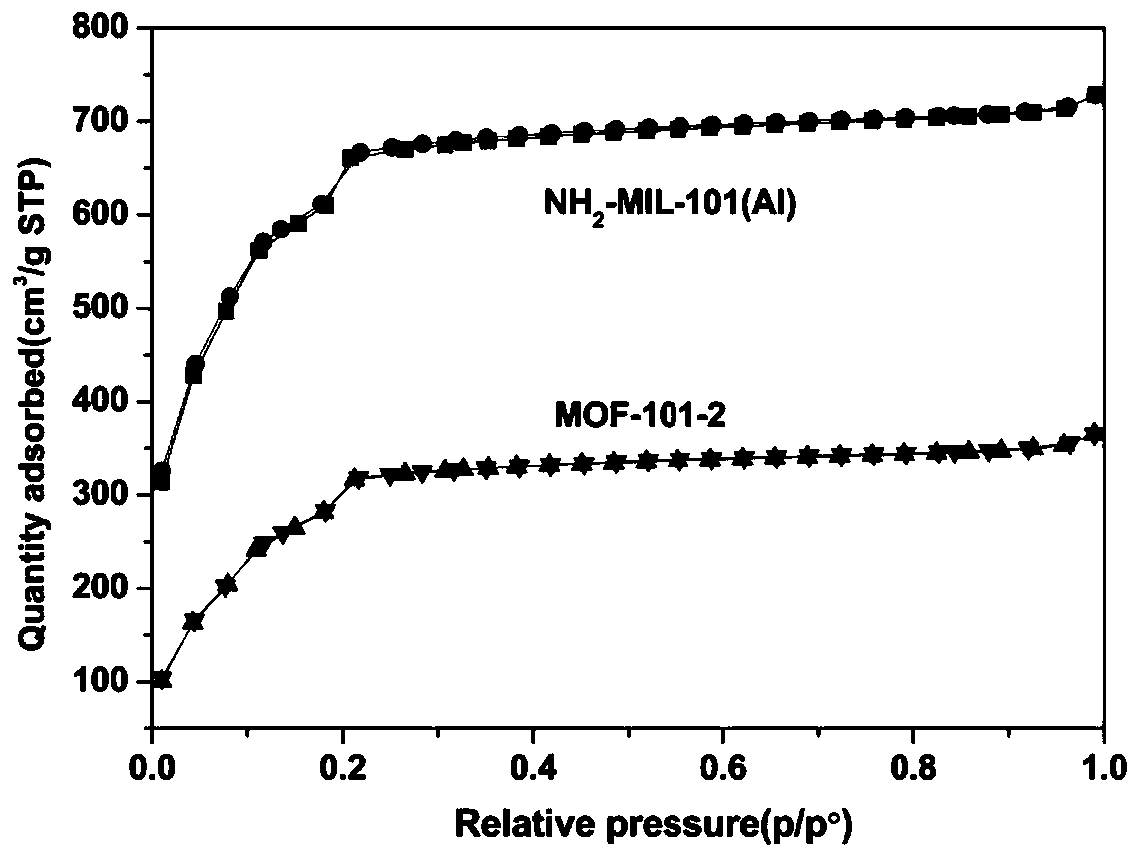
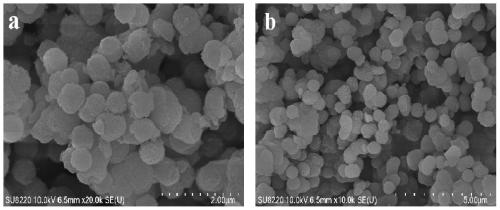
[0041] Embodiment 2 gained NH 2 - PXRD patterns, nitrogen adsorption-desorption isotherms, and SEM images of MIL-101(Al) and MOF-101-2 Figure 1-3 as shown ( image 3 where a is NH 2 - MIL-101 (Al); b is MOF-101-2). After the iridium is immobilized, the MOF still maintains the original crystal structure, but the specific surface area is greatly reduced due to the presence of iridium components in the pores. -NH on organic linker 2 The steric effect of the complexation of the ligand and the metal ion is increased, resulting in a decrease in the crystallinity of the MOF, and there is no regular octahedral structure.
[0042] Table 1 The influence of different iridium solid loads on the production of hydrogen from formic acid
[0043] Example 1 2 3 Iridium theoretical immobilized capacity / (wt.%) 10 15 20 Mass of iridium hydrate / g 0.07 0.11 0.15 Reaction time / min 240 180 195 Gas volume / mL 353 418 356
Embodiment 4-6
[0045] Referring to Example 2, MOF-101-2 with a theoretical loading of iridium of 15 wt.% was synthesized. According to Table 2, pour 20-80mg MOF-101-2 into the flask, and place the flask in an oil bath that has been preheated to 50°C. After 20 minutes, inject 5 mL of 2M formic acid aqueous solution into the flask, start magnetic stirring, and start timing. When the gas production is less than 2 mL after 5 minutes, stop the reaction, and pour water into the flask to exhaust the gas remaining in the device. See Table 2 for the gas production of formic acid decomposition under different catalyst dosages. For Examples 4-7, the content of CO in the reaction product gas is no more than 10ppm, H 2 and CO 2 The concentration ratio is 1:1.
[0046] Table 2 The influence of catalyst dosage on formic acid hydrogen production
[0047] Example 4 2 5 6 Catalyst dosage / mg 20 40 60 80 Reaction time / min 288 180 142 129 Gas volume / mL 386 418 430 43...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com