A kind of isradipine controlled-release tablet and preparation method thereof
A technology of isradipine and tablet compression, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the impact of large-scale production, high residual organic solvents, and long drying time And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
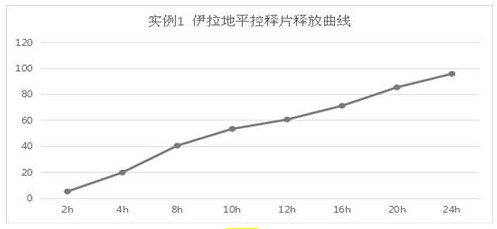
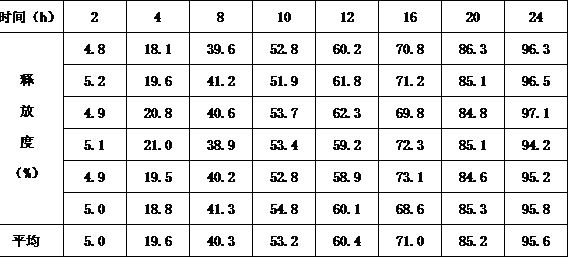
example 1
[0012] The preparation of example 1. isradipine controlled release tablet
[0013] (1) Preparation of double-layer core
[0014] ① drug layer
[0015] Isradipine 5g
[0016] Carbomer 70g
[0017] Povidone 30g
[0018] Silica 1g
[0020] Mix the isradipine, carbomer and povidone in the above prescription amount evenly, prepare a soft material with an appropriate amount of 60-80% ethanol, granulate it with a 18-mesh nylon net, and ventilate and dry the prepared granules at 50°C. Then add silicon dioxide and magnesium stearate, mix well, and set aside.
[0021] ② Booster layer
[0022] Low-substituted hydroxypropyl cellulose 30g
[0023] Cross-linked sodium carboxymethyl starch 60g
[0025] Red Iron Oxide 2g
[0026] Silica 1g
[0028] Mix the low-substituted hydroxypropyl cellulose, cross-linked carboxymethyl starch sodium, sodium chloride, and red iron oxide in the above pres...
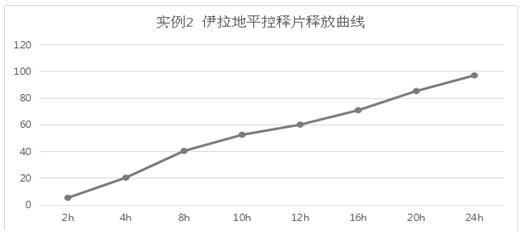
example 2
[0046] The preparation of example 2. isradipine controlled-release tablets
[0047] (1) Preparation of double-layer core
[0048] ① drug layer
[0049] Isradipine 10g
[0050] Carbomer 90g
[0051] Povidone 45g
[0052] Silica 1g
[0054] Mix the isradipine, carbomer and povidone in the above prescription amount evenly, prepare a soft material with an appropriate amount of 60-80% ethanol, granulate it with a 18-mesh nylon net, and ventilate and dry the prepared granules at 50°C. Then add silicon dioxide and magnesium stearate, mix well, and set aside.
[0055] ② Booster layer
[0056] Cross-linked sodium carboxymethyl starch 70g
[0057] Hypromellose 40g
[0059] Red Iron Oxide 2g
[0060] Silica 1g
[0061] Magnesium Stearate 1g
[0062] Mix the hydroxypropylmethyl cellulose, cross-linked sodium carboxymethyl starch, sodium chloride, and red iron oxide in the above prescription amount evenly, prepare sof...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com