Ligand, preparation method thereof, fluorescent probe, and preparation method and application of fluorescent probe
A technology of fluorescent probes and ligands, applied in the fields of fluorescent probes and their preparation, ligands and preparations, can solve problems such as low signal-to-noise ratio and accuracy, achieve good selectivity, improve accuracy and precision , the effect of high measurement sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A ligand of the present invention: 2,2', 2", 2"'-(((4'-(4-((bis(pyridin-2-ylmethyl)amino)methyl)-1H- 1,2,3-triazol-1-yl)-[2,2':6',2"-terpyridine]-6,6'-diyl)bis(methylene))bis(azanetri Base)) tetraacetic acid (abbreviated DATP); DATP has the structural formula of following formula I:
[0058]
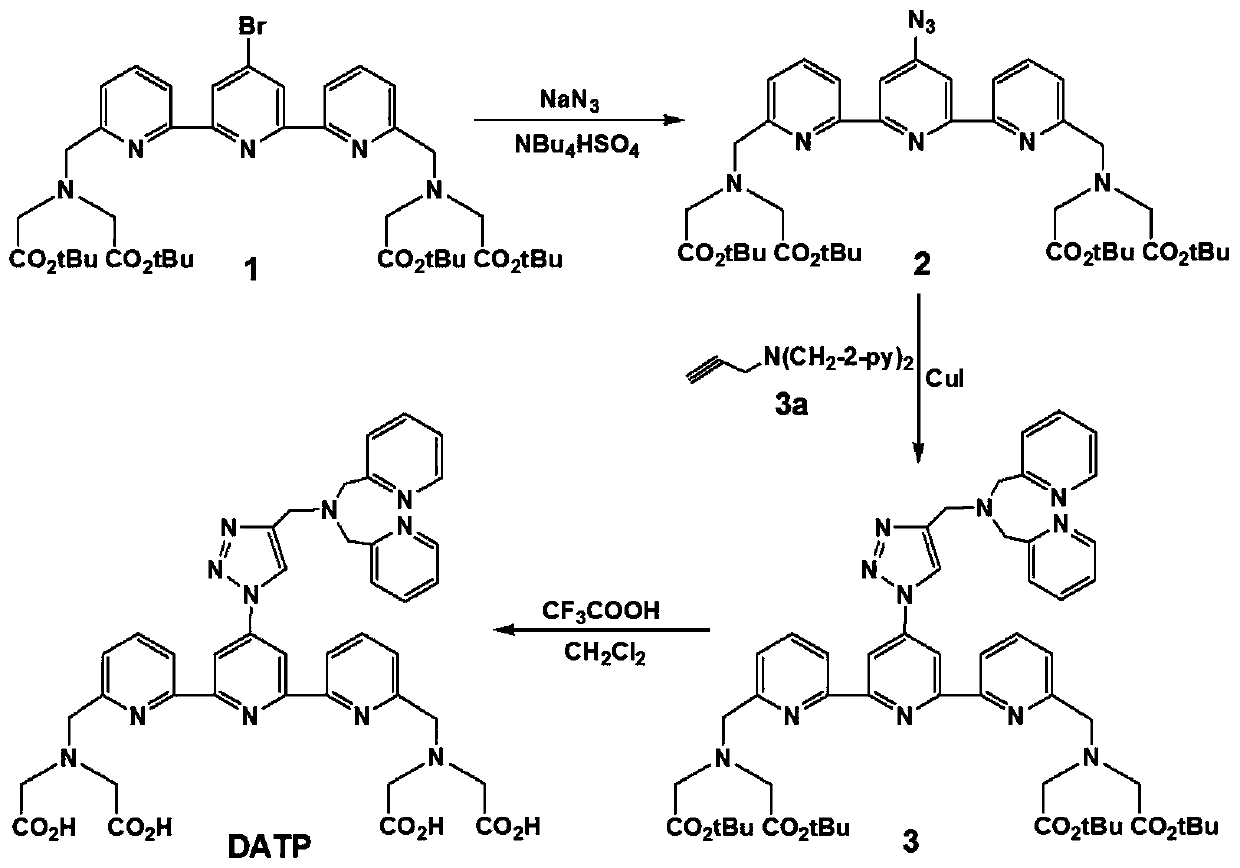
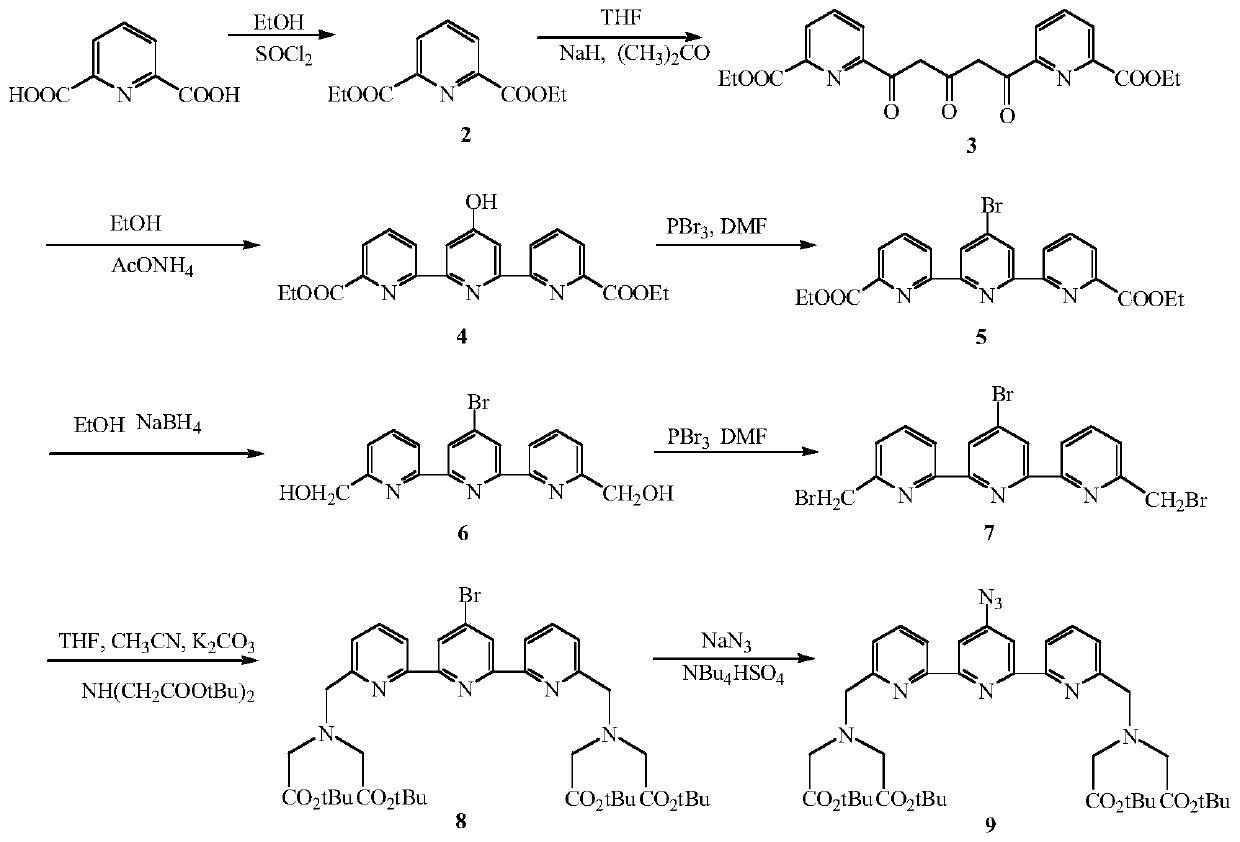
[0059] A synthetic method of the ligand of this embodiment, the synthetic route is as follows image 3 As shown, the specific process is as follows:
[0060] (1) Synthesis of 4'-azido-2,2':6',2"-biterpyridine-6,6"-dimethylaminetetraacetic acid tert-butyl ester (compound 2), the synthetic route is as follows figure 2 Shown:
[0061] 1.1. In a 100mL round bottom flask, add 20mL DMF, 825mg compound 1 (1.0mmol), 650mgNaN 3 (10mmol), 67.8mg tetrabutylammonium bisulfate (0.5mmol), under the protection of argon, reacted at 105°C for 24 hours to obtain the reaction product 1.
[0062] 1.2. Add a small amount of water to the reaction product 1 in step 1.1, extract with methyl tert...
Embodiment 2
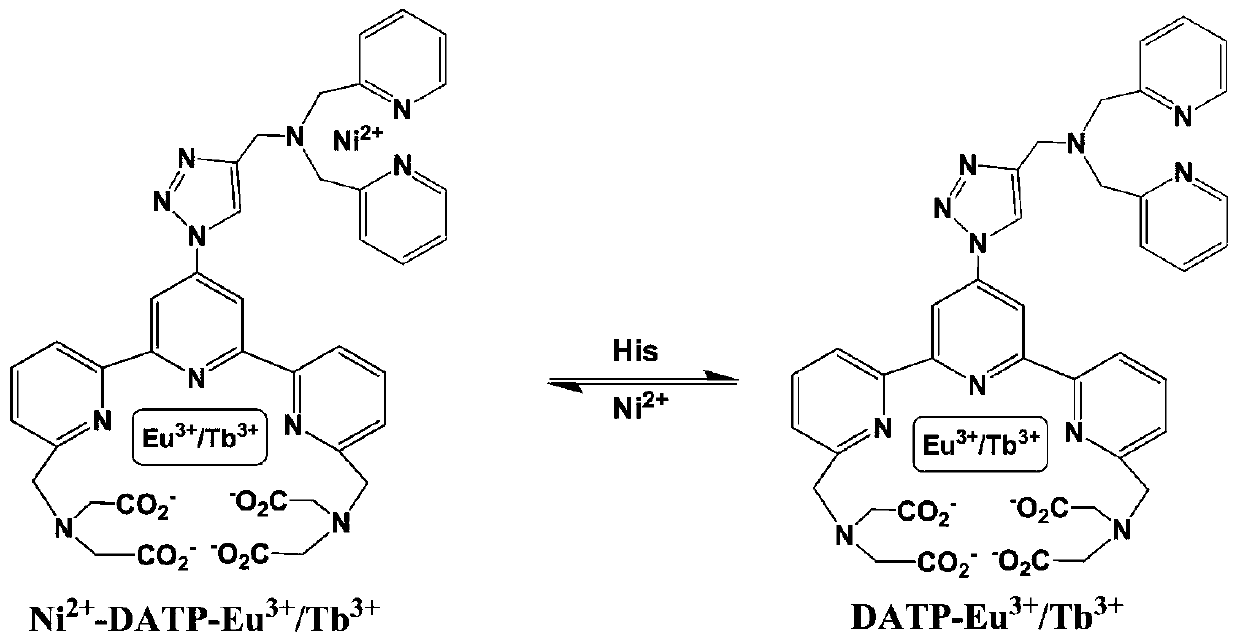
[0090] A kind of fluorescent probe of the present invention: Ni 2+ -DATP-Eu 3+ / Tb 3+ , which has the structural formula of the following formula II:
[0091]
[0092] Ni in this example 2+ -DATP-Eu 3+ / Tb 3+ Prepared by the following method:
[0093] DATP (6.0μmol), EuCl 3 ﹒ 6H 2 O (2.0 μmol) and TbCl 3 ﹒ 6H 2 O (4.0μmol) was dissolved in 6.0mL of 10mM PBS buffer solution and stirred vigorously for 0.5h, then Ni was added 2+ (6.0μmol) after stirring for 0.5h, the probe Ni 2+ -DATP-Eu 3+ / Tb 3+ . Ni 2+ :DATP:Eu 3+ :Tb 3+ =3:3:1:2.
[0094] Fluorescent probe Ni 2+ -DATP-Eu 3+ / Tb 3+ To measure the fluorescence properties:
[0095] (1) Fluorescence spectrum
[0096] Add Ni to the 10mmol / L PBS buffer solution with a pH value of 7.4 2+ -DATP-Eu 3+ / Tb 3+ (Ni 2+ / DATP / Eu 3+ / Tb 3+ =3 / 3 / 1 / 2,C total =10 μM) and histidine concentrations of 0, 0.5, 1, 2, 3, 4, 10, 40, 100, 150, 200, 300, 400, 500, 600, 700, 800, 900, 1000 μM were stirred at 37° C. for ...
Embodiment 3
[0110] A kind of fluorescent probe Ni of embodiment 2 2+ -DATP-Eu 3+ / Tb 3+ For the application of histidine time-resolved fluorescence imaging assay in Hela cells:
[0111] (1) Preparation of AM-DATP-Eu 3+ / Tb 3+ : put Ni 2+ -DATP-Eu 3+ / Tb 3+ DATP-Eu 3+ / Tb 3+ Reaction with bromomethyl acetate to generate AM-DATP-Eu 3+ / Tb 3+ , making it easier for the probe to enter living cells. The specific steps are: dissolve 15.5 μmol DATP in 210 μL anhydrous DMSO, then add 282 μmol (40 μL) triethylamine and 618 μmol (61 μL) bromomethyl acetate, respectively. After stirring at room temperature for 20 hours, centrifuge to remove trace insoluble matter, and then add 5.17 μmol EuCl 3 ·6H 2 O solid and 10.3 μmol TbCl 3 ·6H 2 O, the prepared solution is AM-DATP-Eu 3+ / Tb 3+ , the concentration is about 50mmol / L. Directly diluted with isotonic saline for cell culture.
[0112] (2) will contain 200M AM-DATP-Eu 3+ / Tb 3+ (DATP / Eu 3+ / Tb 3+ =3 / 1 / 2) after culturing Hela cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com