N-type organic semiconductor material based on boron-nitrogen coordination bond and its preparation method and application
An organic semiconductor and coordination bond technology, applied in the fields of semiconductor/solid-state device manufacturing, semiconductor devices, organic chemistry, etc., can solve the problem of difficulty in reducing the LUMO/HOMO energy level, limited n-type molecular design strategies, and low electron mobility, etc. problem, to achieve the effect of favorable charge transport, favorable mass synthesis, and high electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
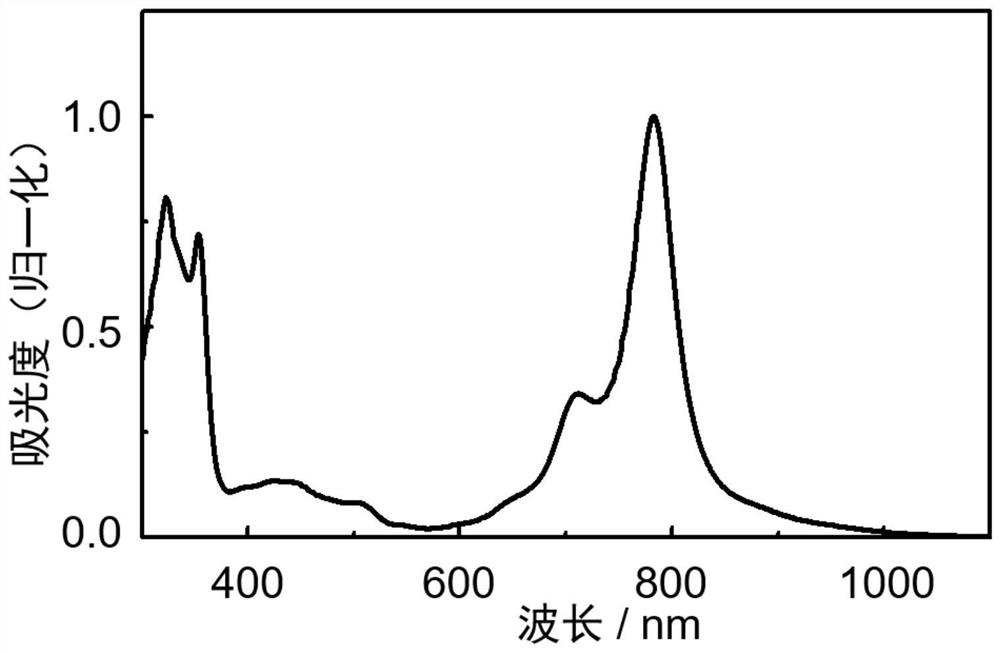
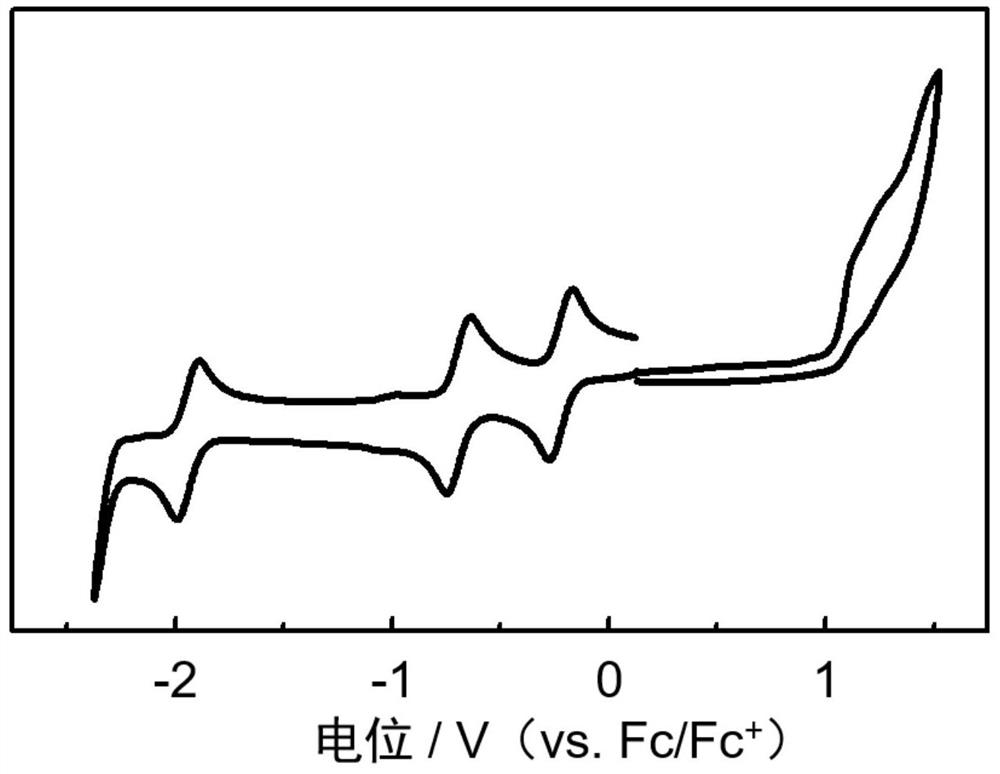
[0068] An n-type organic semiconductor material based on a boron-nitrogen coordination bond, the structural formula is as follows:
[0069]
[0070] Preparation of the above-mentioned n-type organic semiconductor material based on the boron-nitrogen coordination bond:
[0071] Step 1. Accurately weigh 1,3,6,8-tetrabromopyrene (compound 1) (1.00g, 1.93mmol), sodium periodate (3.93g, 18.4mmol) and ruthenium trichloride hydrate into the sealed tube (40mg), then the system was vacuumed, the system was pumped and ventilated several times with argon gas, 45mL of purified acetonitrile and 10mL of primary water were added, the temperature of the system was raised to 120°C, and the reaction was carried out at high temperature and high pressure for 20h. The reaction system was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a solid product, which was ultrasonically washed with water, methanol and chloroform in turn, and filtered with suc...
Embodiment 2
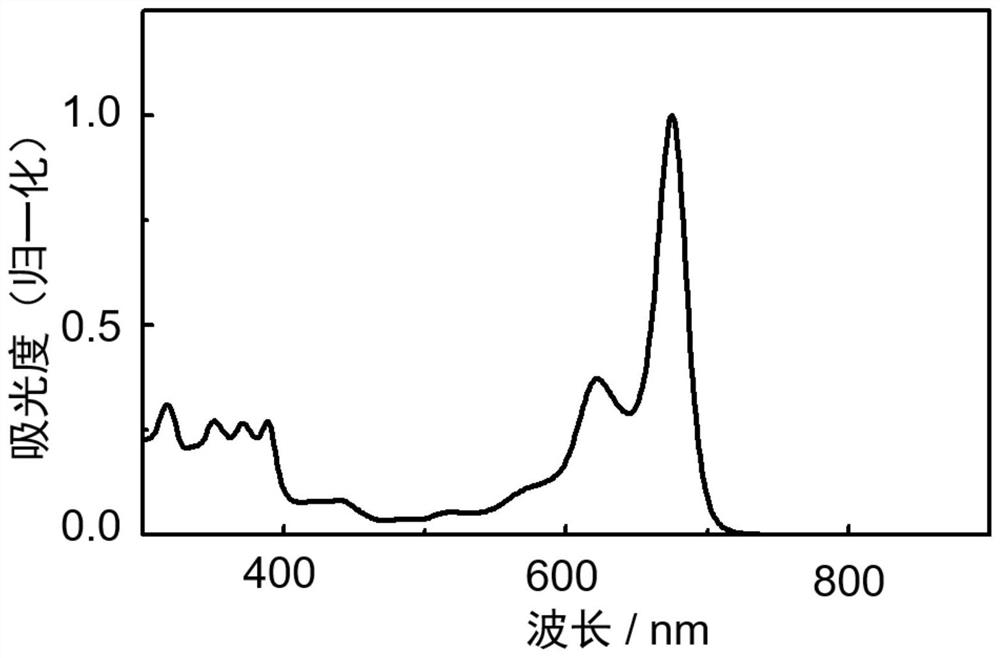
[0084] The structural formula of the n-type organic semiconductor material based on the boron-nitrogen coordination bond is as follows:
[0085]
[0086] The preparation of the above-mentioned n-type organic semiconductor material based on boron-nitrogen coordination bond: add the boranation precursor (16.0mg, 0.012mmol) to the polymerization bottle that has been baked clean, then vacuumize and pass argon to the system. Ventilate several times, add distilled toluene solvent (15mL), and add triethylamine (0.20mL, 1.42mmol) and boron trifluoride ether (0.79mL, 2.84mmol) dropwise under reflux at 120°C for 20min Continue to reflux for 6h after the dropwise addition. The reaction system was cooled to room temperature, concentrated under reduced pressure to remove the solvent, added methanol, ultrasonically settled, filtered the solid crude product obtained, and finally column chromatography (trichloromethane:n-hexane=2:1) to obtain a dark green solid (i.e. based on boron Nitr...
Embodiment 3
[0089] The structural formula of the n-type organic semiconductor material based on the boron-nitrogen coordination bond is as follows:
[0090]
[0091] The preparation of the above-mentioned n-type organic semiconductor material based on boron-nitrogen coordination bonds: add the borylation precursor (11.0mg, 0.007mmol) to the polymerization bottle that has been baked clean, then vacuumize and pass argon to the system. Ventilate several times, add distilled toluene solvent (10mL), and add triethylamine (0.12mL, 0.84mmol) and boron trifluoride diethyl ether (0.46mL, 1.68mmol) dropwise under reflux at 120°C for 20min Continue to reflux for 6h after the dropwise addition. The reaction system was cooled to room temperature, concentrated under reduced pressure to remove the solvent, and the solid was added to methanol, ultrasonically settled, filtered to obtain the solid crude product, and finally column chromatography (dichloromethane:n-hexane=2:1) to obtain a dark green so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com