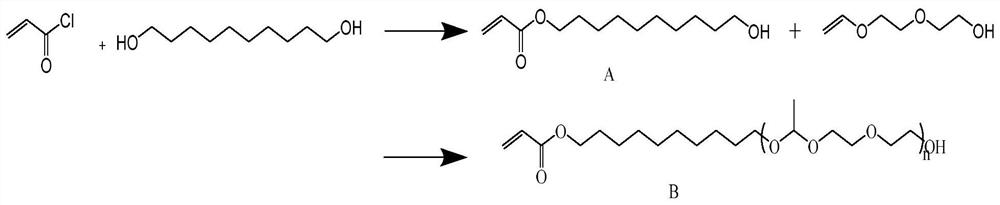
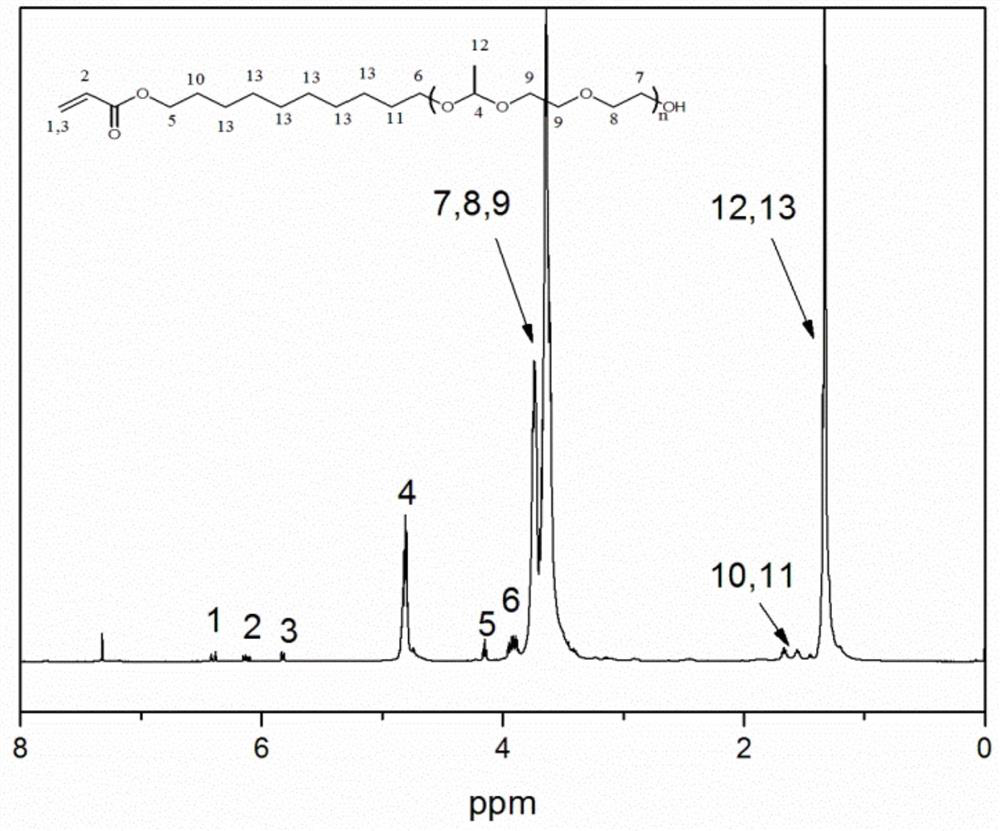
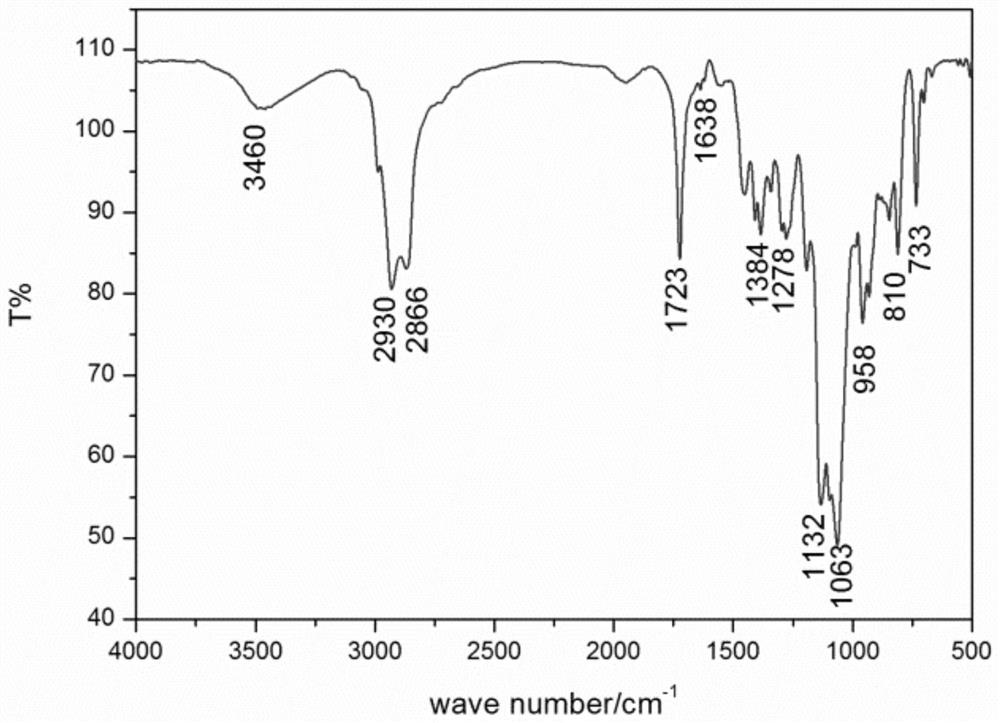
A polymerizable acid-sensitive amphiphilic compound
A technology of amphiphilic compounds and polymeric acids, applied in the preparation of organic compounds, organic chemistry, carboxylate preparation, etc., can solve the problems of poor solubility, poor solubility of nanomaterials, and limit the practical application of nanomaterials, and achieve full reaction Complete, high-yield results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Step 1: Stir and mix 0.192g (1.1mmol) 1,10-decanediol, 0.111g (1.1mmol) triethylamine and 20ml dichloromethane at 5°C at a speed of 600r / min to form the first mixed solution ;
[0049] Step 2: Mix 0.090g (1mmol) of acryloyl chloride and 20ml of dichloromethane into a constant pressure dropping funnel to completely dissolve them to form a second mixed solution;
[0050]Step 3: Pour the second mixed solution obtained in step 2 into the first mixed solution obtained in step 1, stir and mix, and react at a temperature of 25°C and a rotational speed of 1800r / min, and the reaction time is 20h; the reaction ends After filtering to remove the precipitate, dry at 35°C and 0.09Mpa for 10 minutes to obtain the crude product; the crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain the pure product A;
[0051] Step 4: Dissolve the obtained product A and 0.009g (0.05mmol) p-toluenesulfonic acid in 20mol methylene chloride,...
Embodiment 2
[0059] Step 1: Stir and mix 0.074g (1.2mmol) ethylene glycol, 0.141g (1.4mmol) triethylamine and 20ml methylene chloride at 5°C at a speed of 600r / min to form the first mixed solution;
[0060] Step 2: Mix 0.109g (1.2mmol) of acryloyl chloride and 20ml of dichloromethane into a constant pressure dropping funnel to completely dissolve it to form a second mixed solution;
[0061] Step 3: Pour the second mixed solution obtained in step 2 into the first mixed solution obtained in step 1, stir and mix, and react at a temperature of 20°C and a rotational speed of 1800r / min, and the reaction time is 18h; the reaction ends After filtering to remove the precipitate, dry at 35°C and 0.09Mpa for 10 minutes to obtain the crude product; the crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain the pure product A;
[0062] Step 4: Dissolve the obtained product A and 0.009g (0.05mmol) p-toluenesulfonic acid in 20mol methylene chlorid...
Embodiment 3
[0070] Step 1: Stir and mix 0.106g (0.9mmol) 1,6-hexanediol, 0.081g (0.8mmol) triethylamine and 20ml dichloromethane at 5°C at a speed of 600r / min to form the first mixed solution ;
[0071] Step 2: Mix 0.073g (0.8mmol) of acryloyl chloride and 20ml of dichloromethane into a constant pressure dropping funnel to completely dissolve it to form a second mixed solution;
[0072] Step 3: Pour the second mixed solution obtained in step 2 into the first mixed solution obtained in step 1, stir and mix, and react at a temperature of 30°C and a rotational speed of 1800r / min, and the reaction time is 22h; the reaction ends After filtering to remove the precipitate, dry at 35°C and 0.09Mpa for 10 minutes to obtain the crude product; the crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain the pure product A;
[0073] Step 4: Dissolve the obtained product A and 0.009g (0.05mmol) p-toluenesulfonic acid in 20mol methylene chloride,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com