A kind of biotransformation method for synthesizing hexahydropyridazine-3-carboxylic acid
A technology of hexahydropyridazine and biotransformation, applied in the biological field, can solve the problems of high cost, complex process, low stereoselectivity, etc., achieve good technical application and industrialization prospects, mild and easy to control reaction conditions, and specific stereoselectivity sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
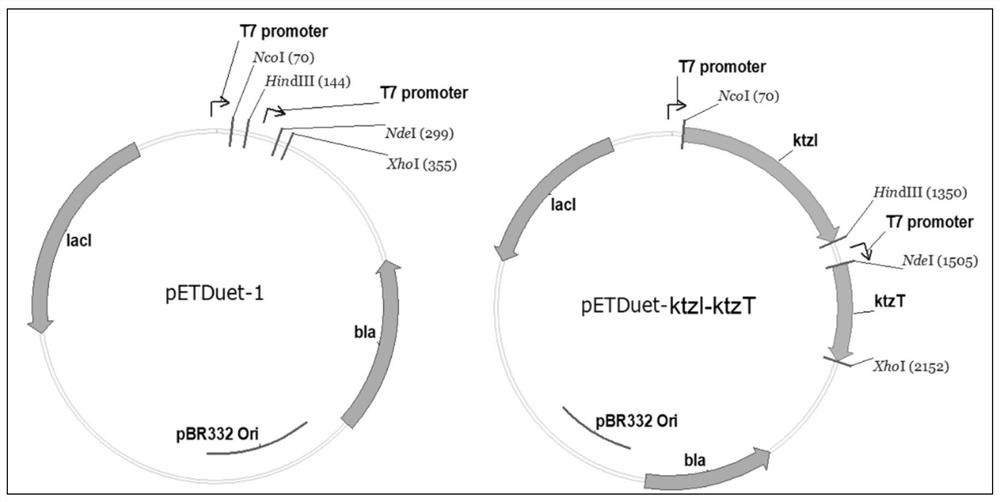
[0045] Construction of a recombinant plasmid containing the gene ktzI encoding L-ornithine hydroxylase and the gene ktzT encoding L-piperazine acid synthase
[0046] According to the amino acid sequences of L-ornithine hydroxylase and L-piperazine acid synthetase, as shown in SEQ ID NO.1 and SEQ ID NO.2 respectively, and codon optimization is carried out according to the codon preferred by Escherichia coli, according to The characteristics of the expression vector pETDuet-1, design PCR primers, design enzyme cutting sites NcoI and HindIII at both ends of the L-ornithine hydroxylase coding gene ktzI, and design enzymes at both ends of the L-piperazine synthase coding gene ktzT The cleavage sites NdeI and XhoI are used to synthesize KtzI and KtzT gene DNA fragments in a fully synthetic manner through conventional genetic engineering operations.
[0047] The DNA fragment of the ktzI gene and the expression plasmid pETDuet-1 were digested with NcoI and HindIII restriction endonucl...
Embodiment 2
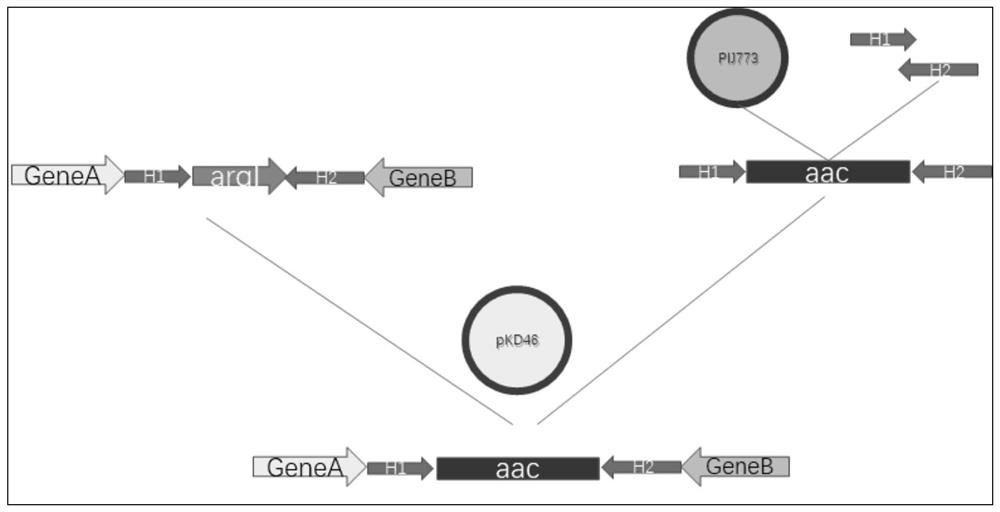
[0049] Construction of host Escherichia coli E.coli BL21-ΔargI that does not consume the substrate L-ornithine itself
[0050] According to the position of ornithine carbamoyltransferase coding gene argI (NCBI database number: AJH11494) on the genome of Escherichia coli E.coli BL21 (DE3), the fragments of homology arms at both ends of argI were designed, and the fragments containing Apramyces The pIJ773 plasmid with the antibiotic resistance marker was used as a template, and the homologous recombination fragment containing the apramycin resistance marker was obtained by PCR, and the gene argI encoding the ornithine carbamoyltransferase was replaced by the conventional E. coli red recombination method Apramycin resistance marker, spread on the LB solid medium plate containing apramycin (final concentration: 50 μg / ml), cultivate overnight at 37°C, and in the colony grown on the plate the next day Randomly pick clones and extract plasmids, sequence them with identification prime...
Embodiment 3
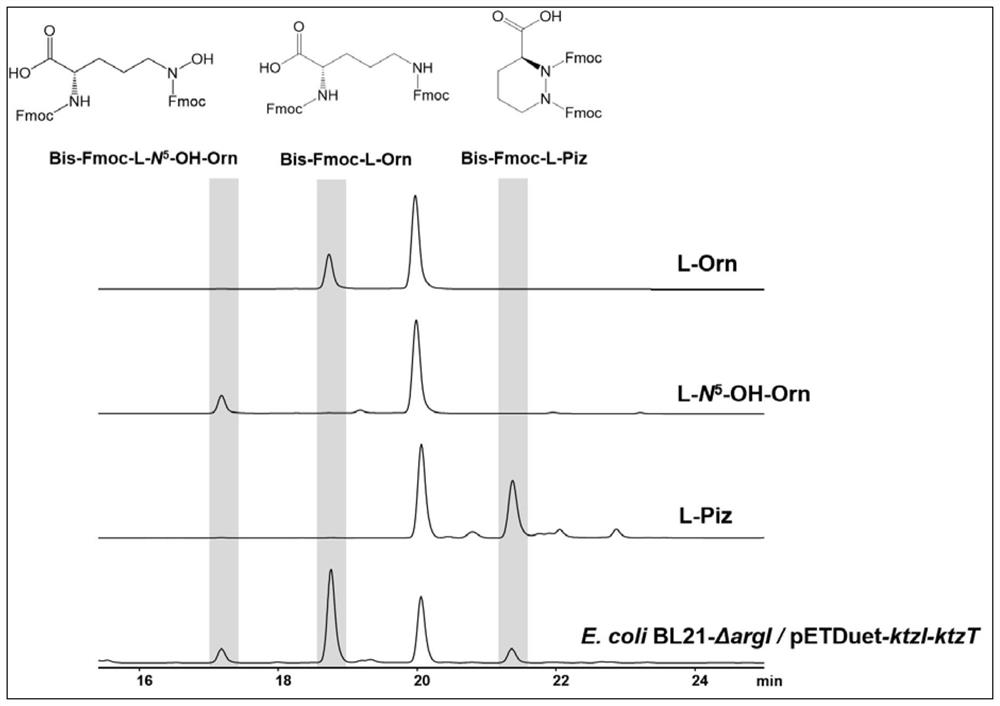
[0055] Construction of genetically engineered bacteria containing genes encoding L-ornithine hydroxylase and L-piperazine acid synthase
[0056] The recombinant plasmid pETDuet-ktzI-ktzT constructed in Example 1 was transformed into the E.coliBL21-ΔargI host bacterium constructed in Example 2, and spread on LB solid medium containing ampicillin (final concentration: 50 μg / ml) On the plate, cultivate overnight at 37°C to construct the genetically engineered bacteria E.coli BL21-ΔargI / pETDuet-ktzI-ktzT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com