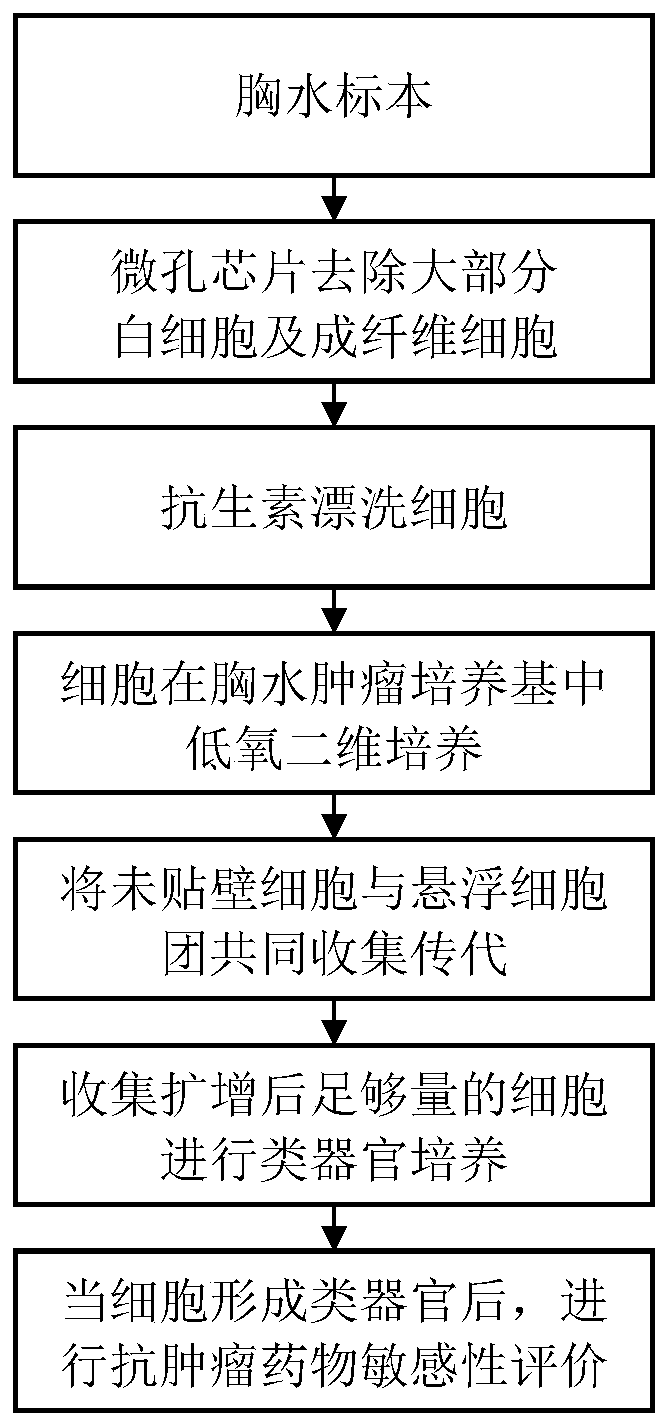
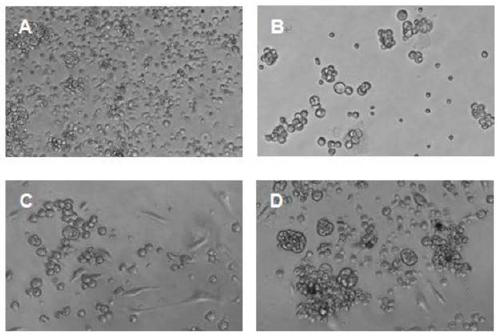
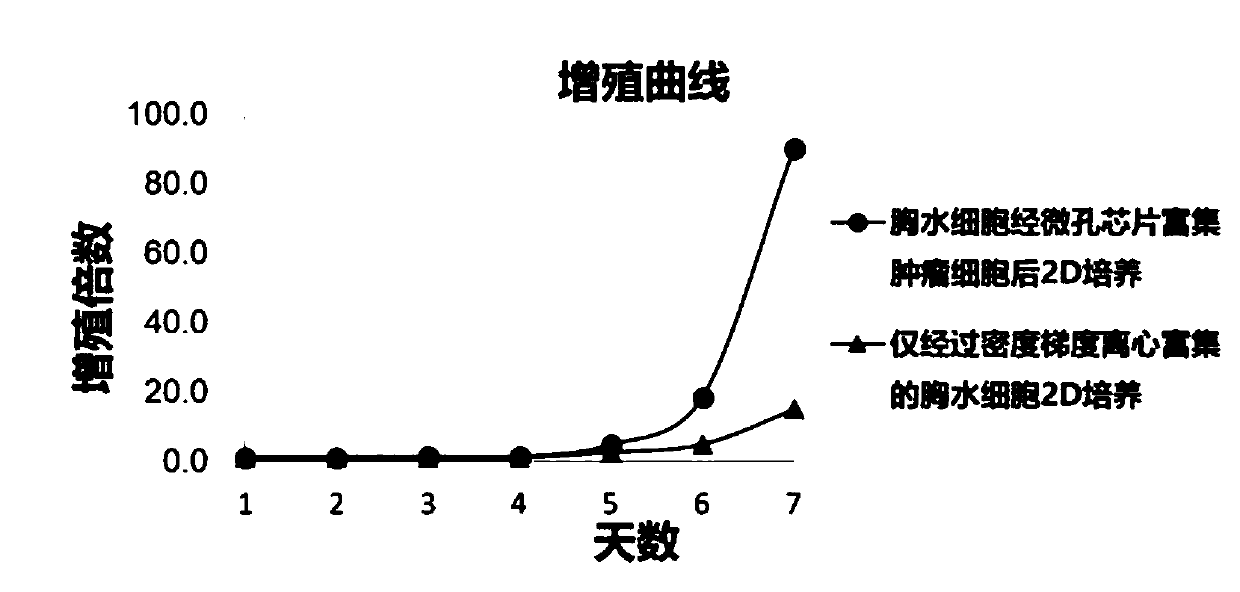
Organoid culture medium and organoid culture method
A culture method and organoid technology, which can be applied to the medium for organoid culture and the field of organoid culture, can solve problems such as low clinical practicability, and achieve the effects of shortening the time required, reducing the cost requirement, and increasing the proliferation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The medium for evaluating antitumor drugs of the present invention includes DMEM medium, pleural fluid supernatant with a volume concentration of 10%, enzymolysis casein with a mass percentage of 1%, and a concentration of 25 ng / kg in the DMEM medium. mL of Hydrocortisone, 2mM L-glutamine, 2μM Insulin, 70nM Transferrin, 0.1nM Cholera toxin, 10μM Y-27632, 0.5ng / mL of EGF, amphotericin B at a concentration of 2.5 μg / mL, penicillin at a concentration of 100 U / mL, and streptomycin at a concentration of 100 μg / mL.
[0042] Preparation of culture medium for antitumor drug evaluation: centrifuge the pleural effusion samples, take the supernatant, and filter through a 0.22 μm filter to obtain the pleural effusion supernatant. 1% Enzymatic Casein, Hydrocortisone at 25ng / mL, L-glutamine at 2mM, Insulin at 2μM, Transferrin at 70nM, Cholera toxin at 0.1nM, Concentration The ratio of Y-27632 at 10 μM, EGF at a concentration of 0.5 ng / mL, amphotericin B at a concentration of 2.5 μg...
Embodiment 2
[0050] The culture medium for antitumor drug evaluation of the present invention includes DMEM / F12 medium, pleural water supernatant with a volume concentration of 40% in DMEM / F12 medium, enzymatic hydrolyzed casein with a mass percentage of 5%, Hydrocortisone at 100 ng / mL, L-glutamine at 10 mM, Insulin at 5 μM, Transferrin at 200 nM, Cholera toxin at 1 nM, Y-27632 at 50 μM, EGF at 100 ng / mL, amphotericin B at 10 μg / mL, penicillin at 100 U / mL, and streptomycin at 100 μg / mL.
[0051] Preparation of culture medium for evaluation of antitumor drugs: centrifuge the pleural effusion samples, take the supernatant, and filter through a 0.22 μm filter to obtain the pleural effusion supernatant. 5% enzymatic casein, hydrocortisone at 100 ng / mL, L-glutamine at 10 mM, Insulin at 5 μM, transferrin at 200 nM, Cholera toxin at 1 nM, The ratios of 50 μM Y-27632, 100 ng / mL EGF, 10 μg / mL amphotericin B, 100 U / mL penicillin, and 100 μg / mL streptomycin were added to DMEM / F12 medium, mixed eve...
Embodiment 3
[0059] The medium for evaluating antitumor drugs of the present invention includes MEM medium, pleural fluid supernatant with a volume concentration of 5%, enzymolysis casein with a mass percentage of 0.5%, and a concentration of 1 ng / mL of hydrocortisone, L-glutamine at 1 mM, Insulin at 1 μM, transferrin at 1 nM, Cholera toxin at 0.1 nM, Y-27632 at 1 μM, 0.5 ng / mL of EGF, amphotericin B at a concentration of 1 μg / mL, penicillin at a concentration of 100 U / mL, and streptomycin at a concentration of 100 μg / mL.
[0060] Preparation of culture medium for evaluation of antitumor drugs: centrifuge the pleural effusion samples, take the supernatant, and filter through a 0.22 μm filter to obtain the pleural effusion supernatant. 0.5% Enzymatic Casein, Hydrocortisone at 1 ng / mL, L-glutamine at 1 mM, Insulin at 1 μM, Transferrin at 1 nM, Cholera toxin at 0.1 nM, Concentration The above components were added in the ratio of 1 μM Y-27632, 0.5 ng / mL EGF, 1 μg / mL amphotericin B, 100 U / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com