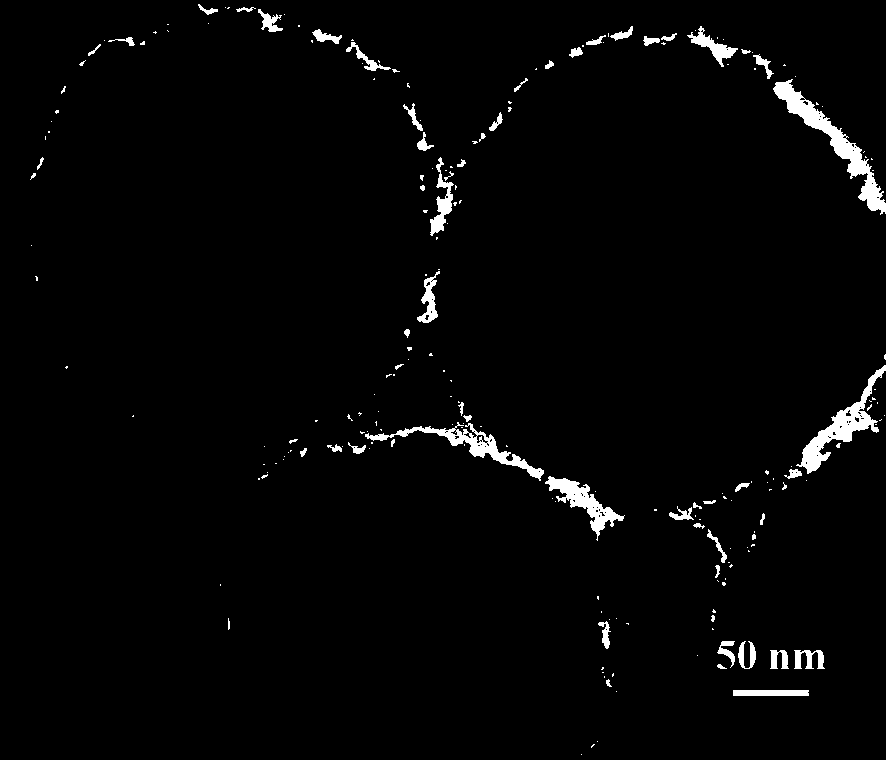
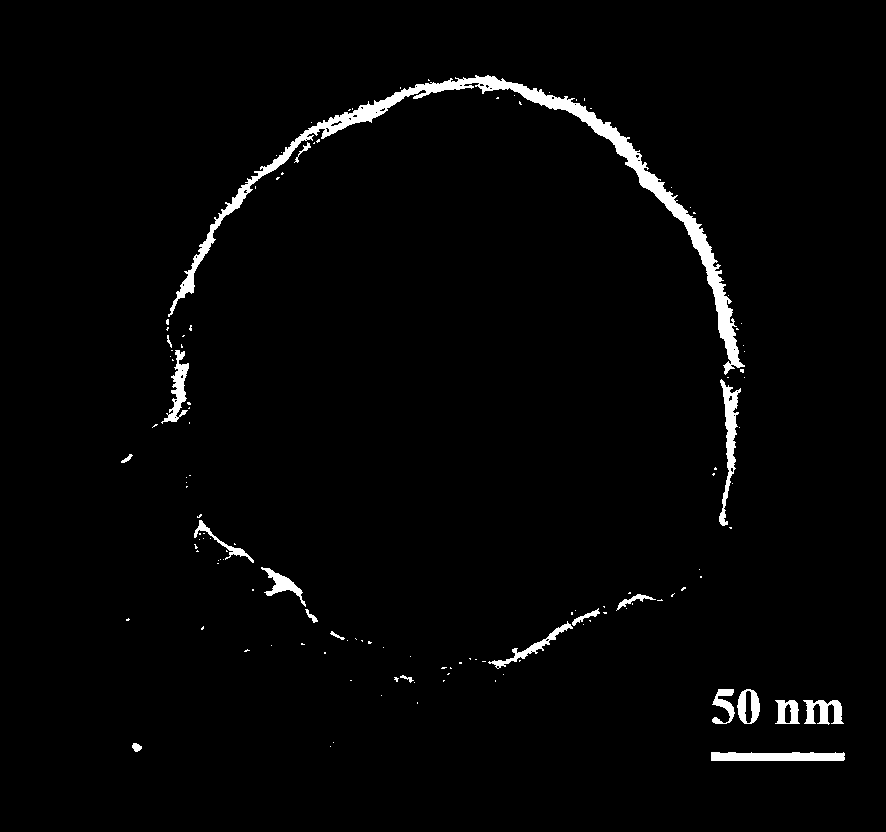
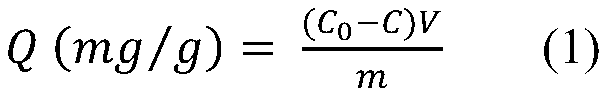Preparation method of phenylboronic acid modified magnetic chitosan and application of phenylboronic acid modified magnetic chitosan in selective separation of shikimic acid
A chitosan, phenylboronic acid technology, applied in selective adsorption, chemical instruments and methods, ion exchange, etc., can solve the problems of strong non-specific adsorption of materials, low product purity, poor method selectivity, etc., and achieve high surface reactivity. , good application prospect, convenient preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of phenylboronic acid modified magnetic chitosan includes the following steps:
[0031] a) According to the solid-to-liquid ratio of 0.5mmol:2.5mmol:0.25mmol:60mL, the FeCl 3 ·6H 2 O, NaAc·3H 2 O and Na 3 C 6 H 5 O 7 ·2H 2 O was dissolved in ethylene glycol, stirred and dissolved, then transferred to a hydrothermal reactor, placed in a blast drying oven at 120℃, after 10 hours of reaction, centrifuged, washed three times with distilled water and absolute ethanol, and dried at 75℃ Dry Nano Fe 3 O 4 solid;
[0032] b) Weigh 1g Fe 3 O 4 Dissolve in 50mL water, stir well; follow Fe 3 O 4 The ratio of mass to chitosan is 1:1. Weigh 1g of chitosan and dissolve it in 300mL of 2% (v / v) acetic acid solution. After stirring, add the solution slowly to contain Fe. 3 O 4 In the aqueous solution, stir for 0.5h; after 0.5h, use 10% (w / v) NaHCO 3 The solution is neutralized to neutral, filtered, washed with water, and dried at 75℃ to obtain Fe 3 O 4 @Chitosan solid;
[0...
Embodiment 2
[0040] A preparation method of phenylboronic acid modified magnetic chitosan includes the following steps:
[0041] a) According to the solid-liquid ratio of 1mmol:2.5mmol:0.4mmol:120mL, the FeCl 3 ·6H 2 O, NaAc·3H 2 O and Na 3 C 6 H 5 O 7 ·2H 2 O was dissolved in ethylene glycol, stirred and dissolved, transferred to a hydrothermal reactor, placed in a blast drying oven at 180℃, after 12 hours of reaction, centrifuged, washed three times with distilled water and absolute ethanol, and dried at 75℃ Dry Nano Fe 3 O 4 solid;
[0042] b) Weigh 1g Fe 3 O 4 Dissolve in 50mL water, stir well; follow Fe 3 O 4 The mass ratio of chitosan is 1:2. Weigh 2g of chitosan and dissolve it in 300mL of 2% (v / v) acetic acid solution. After stirring evenly, add the solution slowly to contain Fe 3 O 4 In the aqueous solution, stir for 2h; after 2h, use 10% (w / v) NaHCO 3 The solution is neutralized to neutral, filtered, washed with water, and dried at 75℃ to obtain Fe 3 O 4 @Chitosan solid;
[0043] c) Pre...
Embodiment 3
[0050] A preparation method of phenylboronic acid modified magnetic chitosan includes the following steps:
[0051] a) According to the solid-to-liquid ratio 1.5mmol:3.5mmol:0.5mmol:100mL, the FeCl 3 ·6H 2 O, NaAc·3H 2 O and Na 3 C 6 H 5 O 7 ·2H 2 O was dissolved in ethylene glycol, stirred and dissolved, then transferred to a hydrothermal reactor, placed in a blast drying oven at 160℃, after 12 hours of reaction, centrifuged, washed three times with distilled water and absolute ethanol, and dried at 75℃ Dry Nano Fe 3 O 4 solid;
[0052] b) Weigh 1g Fe 3 O 4 Dissolve in 50mL water, stir well; follow Fe 3 O 4 The mass ratio to chitosan is 1:3. Weigh 3g of chitosan and dissolve it in 300mL of 2% (v / v) acetic acid solution. After stirring, add the solution slowly containing Fe 3 O 4 In the aqueous solution, stir for 1.5h; after 1.5h, use 10% (w / v) NaHCO 3 The solution is neutralized to neutral, filtered, washed with water, and dried at 75℃ to obtain Fe 3 O 4 @Chitosan solid;
[0053] c)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com