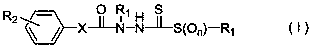N-substituent group-N-(substituted phenylaminocarbonyl)-1-substituted sulfonylthioformylhydrazide derivatives and application thereof
A technology of sulfonylthiocarbohydrazide and anilinoformyl, applied in N-substituent-N-(substituted anilinoformyl)-1-substituted sulfonylthiocarbohydrazide derivatives and their application fields , can solve the problems of increased drug resistance of bacteria, decreased control effect, pesticide residues, etc., and achieve excellent antibacterial activity, simple synthesis method, and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of N-methyl-N-(phenylcarbamoyl)-1-methylthiocarbohydrazide (compound number a):
[0043] (1) The preparation of methyl anilate intermediate:
[0044] Throw aniline (20g, 0.21mol), potassium carbonate (32.65g, 0.24mol) and dichloromethane (100-150mL) in a 250mL three-necked flask, slowly add methyl chloroformate (24.35g, 0.26mol ) after reaction at room temperature for 3-5h. After stopping the reaction, the reaction system was washed with water, separated, dried, filtered with suction and precipitated under reduced pressure to obtain 28.65 g of methyl anilinate, with a yield of 88.25%.
[0045] (2) Preparation of anilinoformohydrazide intermediate:
[0046] Throw methyl aniline formate (15g, 0.1mol) and 80% hydrazine hydrate (62.09g, 1.0mol) in a 100mL three-necked round-bottomed flask, heat up to reflux for 36 hours, cool in an ice bath to precipitate a white solid, and filter with suction The crude product of aniloformyl hydrazide was obtained...
Embodiment 2
[0051] Example 2: Preparation of N-ethyl-N-(phenylcarbamoyl)-1-ethylthiocarbothiohydrazide (compound number is b)
[0052]Step (1)-(3) is the same as embodiment 1
[0053] (4) Preparation of N-ethyl-N-(phenylcarbamoyl)-1-ethylthiocarbohydrazide:
[0054] Throw N-(anilinoyl) potassium dithioformate (1g, 4.32mmol), potassium carbonate (0.6g, 4.32mmol), potassium iodide (62.55mg, 0.38mmol) and 25mL of water in a 50mL three-neck round-bottomed flask, Stir at room temperature until the solid is completely dissolved, slowly add diethyl sulfate (0.76g, 4.90mmol) dropwise, stir at room temperature (20°C) for 12h to end the reaction, filter the system with suction, wash the filter cake with petroleum ether and recrystallize it with absolute ethanol 0.81 g of white solid was obtained, with a yield of 75.38%;
Embodiment 3
[0055] Example 3: Preparation of N-benzyl-N-(phenylcarbamoyl)-1-benzylthiocarbohydrazide (compound number is c)
[0056] Step (1)-(3) is the same as embodiment 1
[0057] (4) Preparation of N-benzyl-N-(phenylcarbamoyl)-1-benzylthiocarbohydrazide:
[0058] Throw N-(anilinoyl) potassium dithioformate (0.325g, 1.22mmol), potassium carbonate (0.169g, 1.22mmol), potassium iodide (0.022g, 0.135mmol) and 25mL of water in a 50mL three-neck round bottom flask , stirred at room temperature until the solid was completely dissolved, slowly added benzyl chloride (0.31g, 2.45mmol) dropwise, stirred at room temperature (20°C) for 12h to end the reaction, filtered the system with suction, washed the filter cake with petroleum ether and recrystallized with absolute ethanol 0.372g of white solid was obtained, yield 74.54%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com