Streptococcus salivarius and application thereof to preparation of medicines for treatment of inflammatory bowel diseases
A technology for Streptococcus salivarius and inflammatory bowel disease, applied in the biological field, can solve the problems of poor understanding of strains, shortage of healthy donors, and in-depth research on intestinal flora, so as to reduce intestinal mucosal damage and improve intestinal inflammation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Isolation and identification of Streptococcus salivarius
[0024] Take feces samples from healthy people, make a gradient dilution of 10^-1 to 10^-10 with sterilized water, plate on BHI solid medium with sterile glass rods, and incubate at 37° for 2 to 3 days.
[0025] Use a sterile inoculation loop to pick a single colony. After purification and passage for 3 times, pick a single colony and transfer it into 5ml of BHI liquid medium, cultivate overnight at 37°C take 1mL of the bacterial solution, centrifuge at 12000g / min for 10min, discard the supernatant, and use the whole Bacterial DNA was extracted using the EasyPure GenomicDNA Kit (EE101).
[0026] PCR amplification: amplification reaction system:
[0027] Amplification system (50μL):
[0028] 2×EasyTaq PCR SuperMix 25 μL DNA template 2μL pre-primer 1μL back primer 1μL Ultra-pure water 21μL
[0029] Primer sequence:
[0030] Front primer, 27F: 5'-AGAGTTTGATC...
Embodiment 2
[0035] Example 2: Establishment and grouping of animal models
[0036] According to the inoculum size of 1:10, inoculate the logarithmic growth of Streptococcus salivarius in sterilized BHI medium, culture overnight at 37°C, centrifuge at 3000G / min for 10min, discard the supernatant, and resuspend with PBS. Adjust to 10^7CFU / mL.
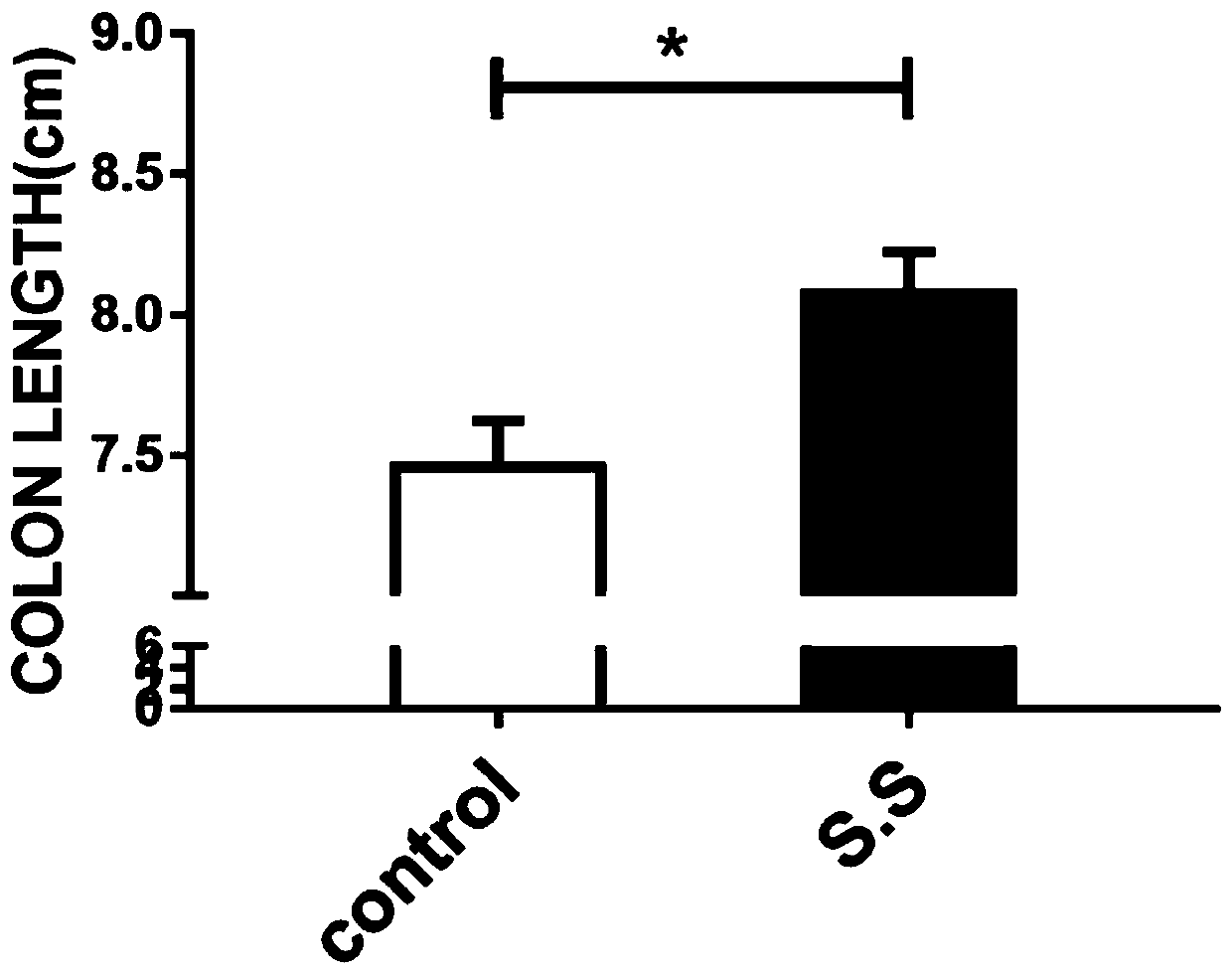
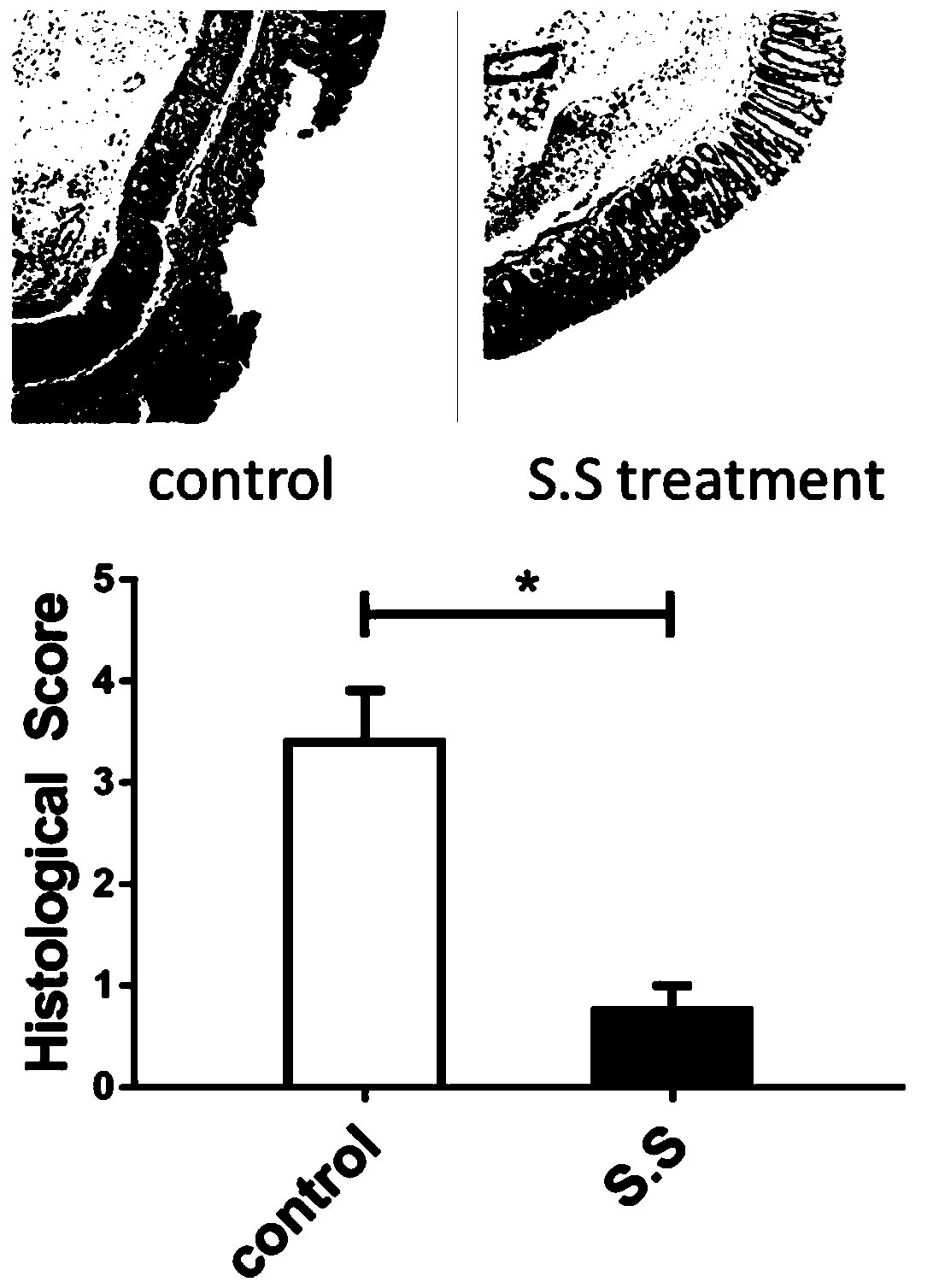
[0037] Male C57 / B6 mice, weighing 22-27g, 8 weeks old, were randomly divided into Control group and S.S group, with 10 mice in each group, and were raised in the SPF environment of a university experimental animal center. Animals were acclimatized for one week before dosing. Both groups were given 2.5% dextran sulfate (mpbio, 9011-18-1) free drinking water for 6 days. The Control group was given 0.2 mL / bird every day for 3 consecutive days; the S.S group was given 0.2 mL / bird of Streptococcus salivarius resuspended in PBS for 3 consecutive days. After overnight starvation, the mice were sacrificed by cervical dislocation, the intestinal length was...
Embodiment 3
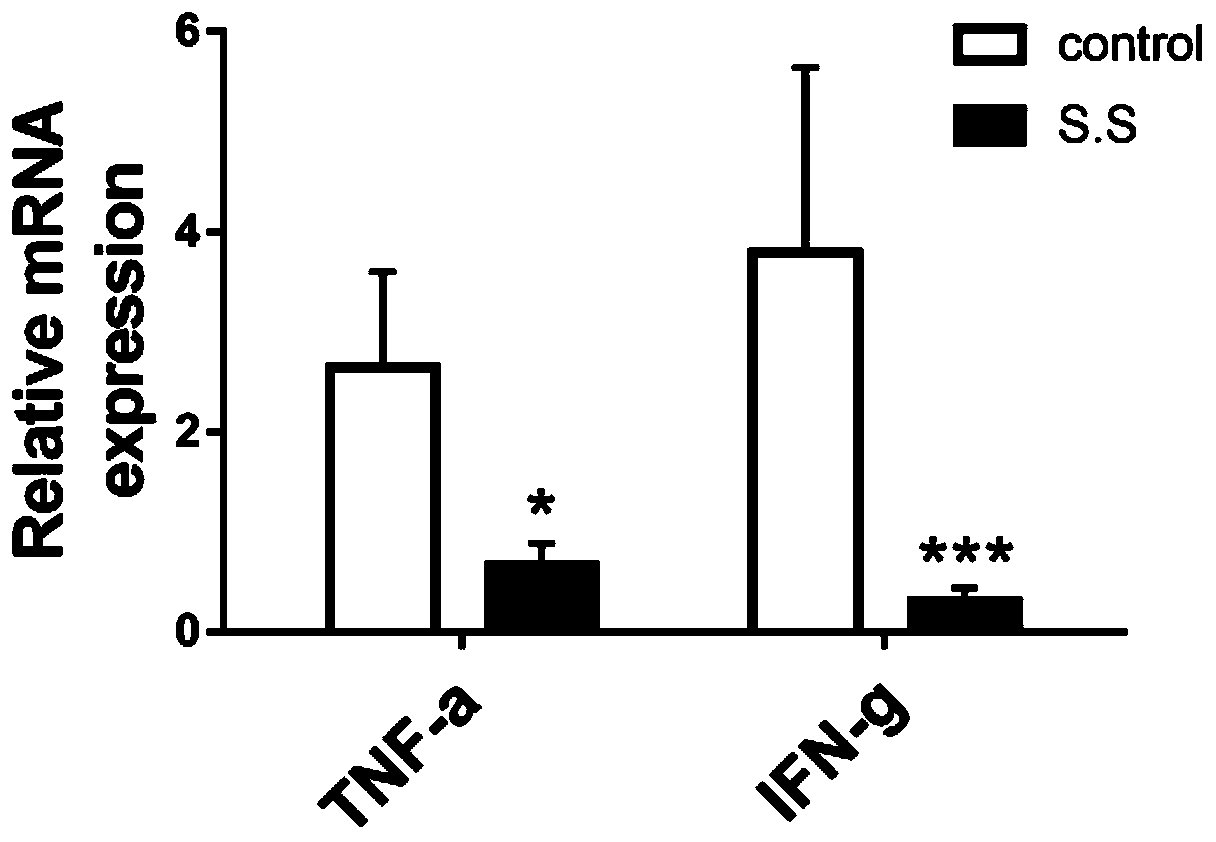
[0038] Example 3: qPCR detection of intestinal inflammatory factors
[0039] Intestinal specimens preserved at -80° were taken, ground in liquid nitrogen, and total RNA was extracted using the TRIzol method, and cDNA was reverse-transcribed using TransScript Two-Step RT-PCR SuperMix (AT401).
[0040] Quantitative PCR amplification:
[0041] Amplification reaction system: Amplification system (20μL):
[0042] TransStart Green qPCR SuperMix 10 μL DNA template 2μL pre-primer 1μL back primer 1μL Ultra-pure water 6μL
[0043] Primer sequence:
[0044] mouse-TNF-a-F CCCTCACACTCAGATCATTCTTCT mouse-TNF-a-R GCTACGACGTGGGCTACAG mouse-IFN-g-F ATGAACGCTACACACTGCATC mouse-IFN-g-R CCATCCTTTTGCCAGTTCCTC mouse-GAPDH-F AGGTCGGTGTGAACGGATTTG mouse-GAPDH-R TGTAGACCATGTAGTTGAGGTCA
[0045] Amplification program: 95°15min; (95°30S, 60°30S)X40 cycle.
[0046] Data analysis and calculation: According t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com