Novel compound of kojic acid and furan and preparation method thereof
A technology of compounds and kojic acid derivatives, applied in organic chemistry and other fields, can solve problems such as screening obstacles of biologically active molecules, and achieve the effect of rich variety, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method of a novel kojic acid and furan compound, comprising the steps of dissolving kojic acid derivative II, (Z)-bromonitroalkene III and bifunctional tertiary thiocarbamide catalyst A in a reaction solvent, adding Alkali, stirring reaction, after the reaction is completed, adopt column chromatography to separate and purify to obtain the compound product with structural formula I, and its reaction structural formula is as follows:
[0040]
[0041] Preferably, the reaction temperature in the stirring reaction process is 10°C-35°C. Preferably, the reaction time is 16-96 hours.
[0042] Preferably, the molar ratio of kojic acid derivative II to (Z)-bromonitroalkene III is 1:1.05.
[0043] Preferably, the reaction solvent is one or a combination of toluene, mesitylene, dichloromethane, chloroform, acetonitrile, tetrahydrofuran, methanol, ethanol or 1,4-dioxane. Further preferably, the reaction solvent is toluene.
[0044] Preferably, the amount of the ...
Embodiment 1
[0048] Embodiment 1: synthetic compound (I-a)
[0049]
[0050]Add 2-(tert-butyldimethylsiloxymethyl)-5-hydroxy-4H-pyran-4-one (Ⅱ) (51.2mg, 0.2mmol) into a rigid glass tube, (Z)- β-bromo-β-nitrostyrene (Ⅲ), bifunctional tertiary thiocarbamide catalyst A (14.4mg, 0.04mmol), and add 2mL toluene to dissolve completely, then add sodium bicarbonate (84mg, 1.0mmol), react The mixture was reacted at room temperature for 48 hours. After the reaction was complete, the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography to obtain compound I-a (white solid, yield 99%).
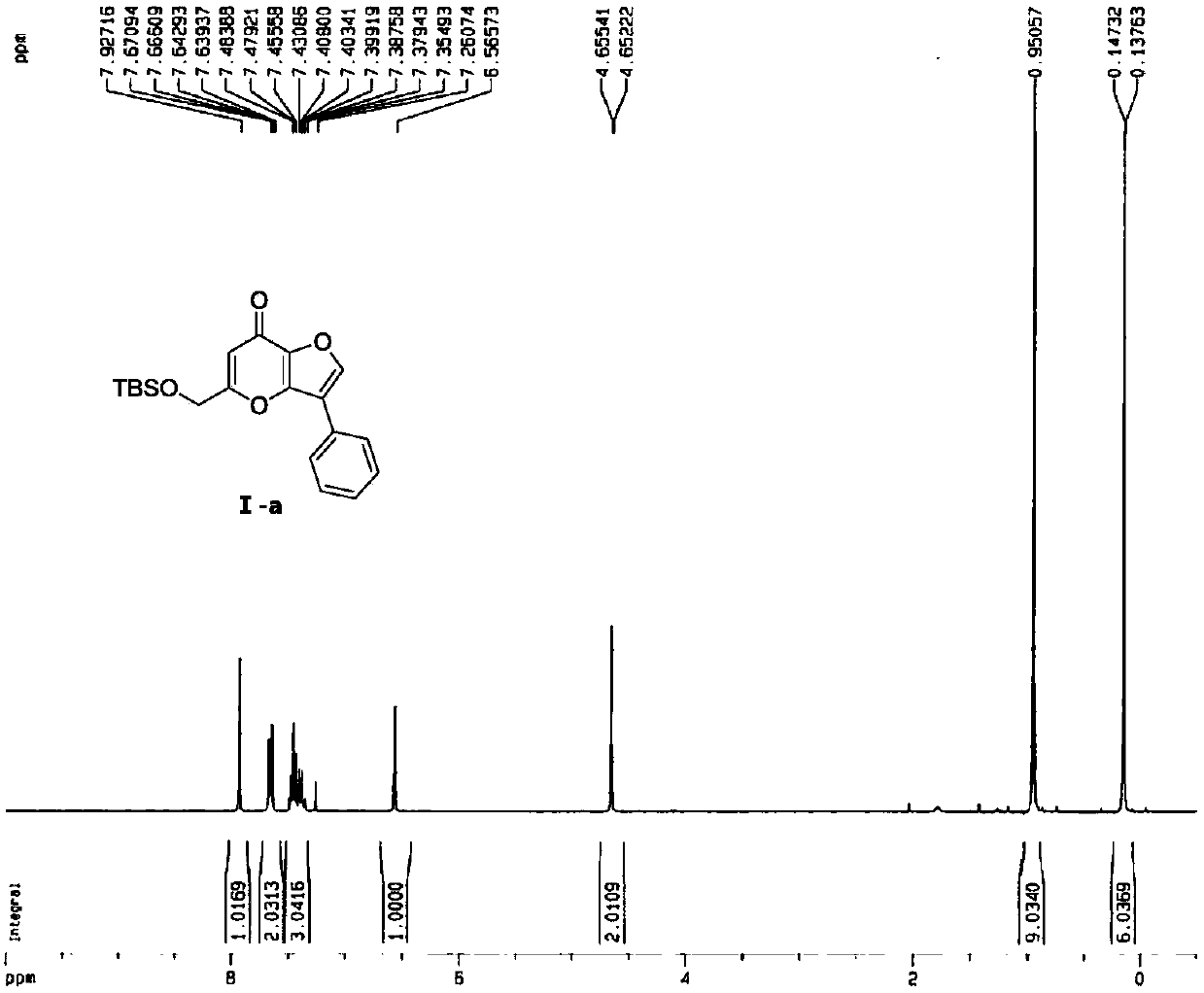
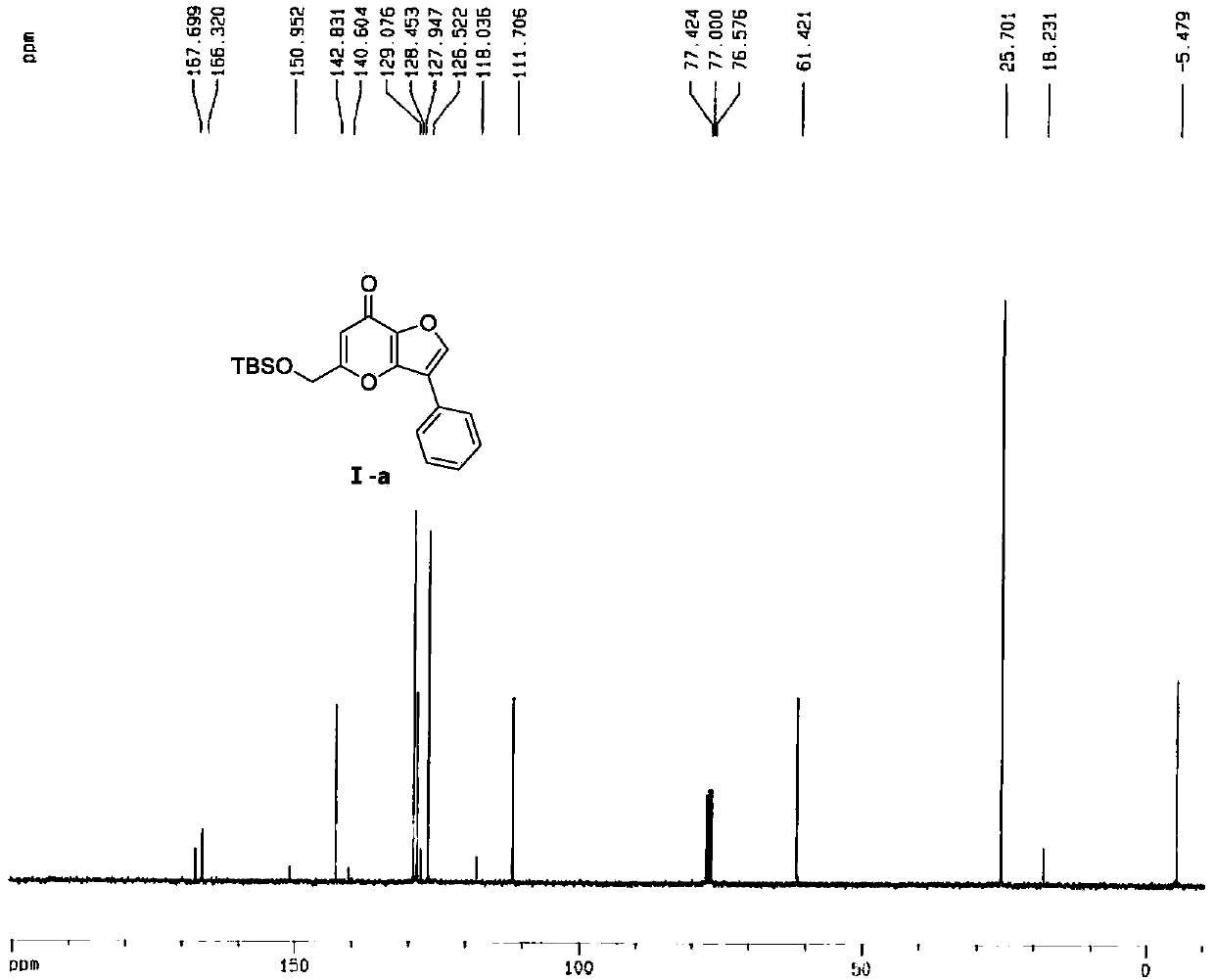
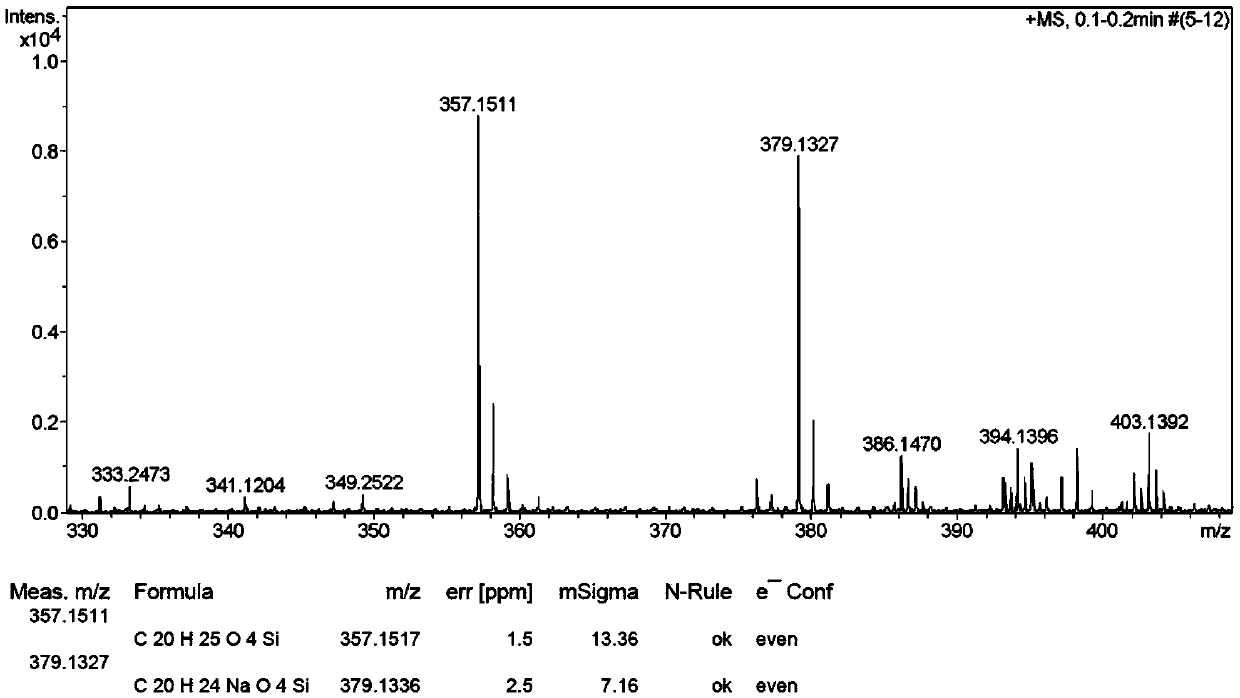
[0051] The hydrogen spectrum, carbon spectrum, mass spectrum and molecular structure of the obtained compound I-a are as follows: Figure 1 to Figure 4 As shown, the melting point, hydrogen spectrum, carbon spectrum and mass spectrum data of the obtained compound I-a are as follows:
[0052] Melting point (m.p.):126.7-127.7℃, ...
Embodiment 2
[0053] Embodiment 2: synthetic compound (I-b)
[0054]
[0055] Add 2-(tert-butyldimethylsiloxymethyl)-5-hydroxy-4H-pyran-4-one (Ⅱ) (51.2mg, 0.2mmol) into a rigid glass tube, (Z)- β-bromo-4-methoxy-β-nitrostyrene (Ⅲ), bifunctional tertiary thiourea catalyst A (14.4mg, 0.04mmol), and add 2mL toluene to dissolve completely, then add sodium bicarbonate (84mg , 1.0mmol), the reaction mixture was reacted at room temperature for 52 hours. After the reaction was complete, the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography to obtain compound I-b (white solid, yield 92%).
[0056] The melting point, hydrogen spectrum, carbon spectrum and mass spectrum data of obtained compound I-b are as follows:
[0057] Melting point (m.p.):175.7-177.0℃, 1 H NMR (300MHz, CDCl 3 )δ7.85(s,1H),7.67–7.43(m,2H),7.08–6.88(m,2H),6.55(s,1H),4.64(s,2H),3.84(s,3H),0.95 (s,9H),0.14(s,6H). 13 CNMR (75MHz, CDCl3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com