Method of recycling valuable metals in waste lithium cobalt oxide batteries through ion exchange method
An ion exchange method and a valuable metal technology, which is applied in the field of ion exchange method to recover valuable metals in waste lithium cobalt oxide batteries, can solve the problems of complicated metal separation process, difficult filtration by alkali leaching method, incomplete separation, etc. The process is short, the evaporative crystallization process is omitted, and the impurity removal time is short.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
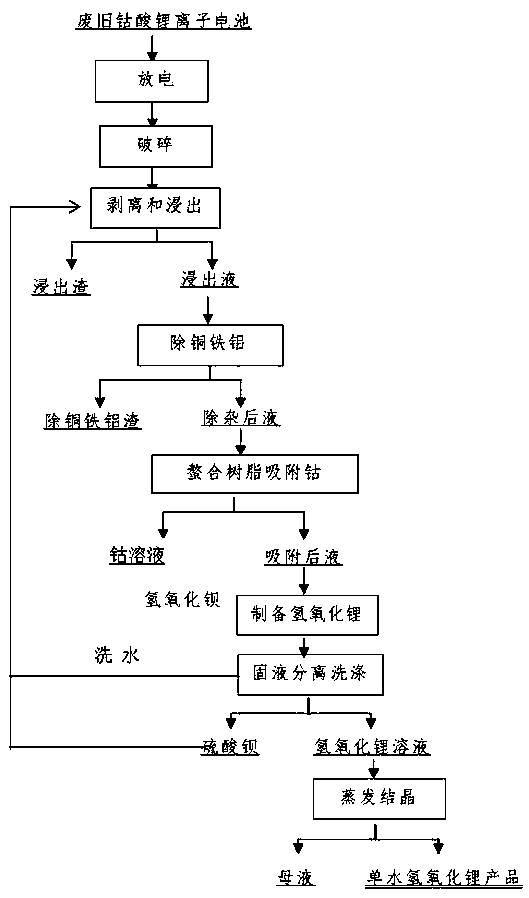
[0022] Collect waste lithium cobaltate batteries, disassemble them manually, discharge them in 5% sodium chloride solution, and crush them mechanically. Weigh 1500g of anhydrous sodium sulfite and dissolve them for later use; add 15L of 1.8M sulfuric acid into the reactor and heat to 50-65°C ;Weigh 5kg of crushed waste lithium-ion battery powder and add it, stir at a low speed, slowly add sulfuric acid to adjust the pH value of the solution to about 1.0, add sodium sulfite solution after 10 minutes, react for 30-100 minutes after completion, and separate the solid and liquid;
[0023] Raise the temperature of the leaching solution to 80-90°C, stop heating, add 50g / L lithium hydroxide solution to adjust the pH value of the solution to 2.0, add 10g of cobalt powder, react for 15 minutes, add 100mL of 30% hydrogen peroxide, and react for 20 minutes, then add lithium hydroxide solution to adjust The pH value of the solution reaches 4.5, reacts for 30min, filters and washes;
[002...
Embodiment 2
[0027] Collect waste lithium cobalt oxide batteries, manually disassemble them, discharge them in 5% sodium chloride solution, and mechanically crush them. Add 15L of 2M sulfuric acid to the reactor, raise the temperature to 50-65°C, weigh 5kg of the crushed waste lithium-ion battery and add it, soak for 10min, stir at a low speed, measure a certain amount of 98% concentrated sulfuric acid, and slowly add it to the reaction vessel. Adjust the pH value of the solution to about 1.0, add 3.0L of 30% hydrogen peroxide, react for 30-100min after the dropwise addition, and filter;
[0028] Raise the temperature of the leaching solution to 85°C, add 50g / L lithium hydroxide solution to adjust the pH value of the solution to 1.8, add 12g of cobalt powder, react for 15 minutes, add 30% hydrogen peroxide 80mL, and react for about 20 minutes, then add 30g / L lithium hydroxide solution to adjust the solution pH value to 4.5, react for 30min, filter and wash;
[0029] The chelating resin D4...
Embodiment 3
[0032] Collect waste lithium cobalt oxide batteries, manually disassemble them, discharge them in 5% sodium chloride solution, and mechanically crush them. Add 15L of 2M sulfuric acid to the reactor, raise the temperature to 50-65°C, weigh 5kg of the crushed waste lithium-ion battery and add it, soak for 10min, stir at a low speed, measure a certain amount of 98% concentrated sulfuric acid, and slowly add it to the reaction vessel. Adjust the pH value of the solution to about 1.0, add 2.8L of 30% hydrogen peroxide, react for 30-100min after the dropwise addition, and filter;
[0033] Raise the temperature of the leaching solution to 85°C, add 50g / L cobalt hydroxide solution to adjust the pH value of the solution to 1.8, add 12g of cobalt powder, react for 15 minutes, add 30% hydrogen peroxide 80mL, and react for about 20 minutes, then add 35g / L cobalt hydroxide solution to adjust the solution pH value to 4.5, react for 30min, filter and wash;
[0034] Chelating resin D402 col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com