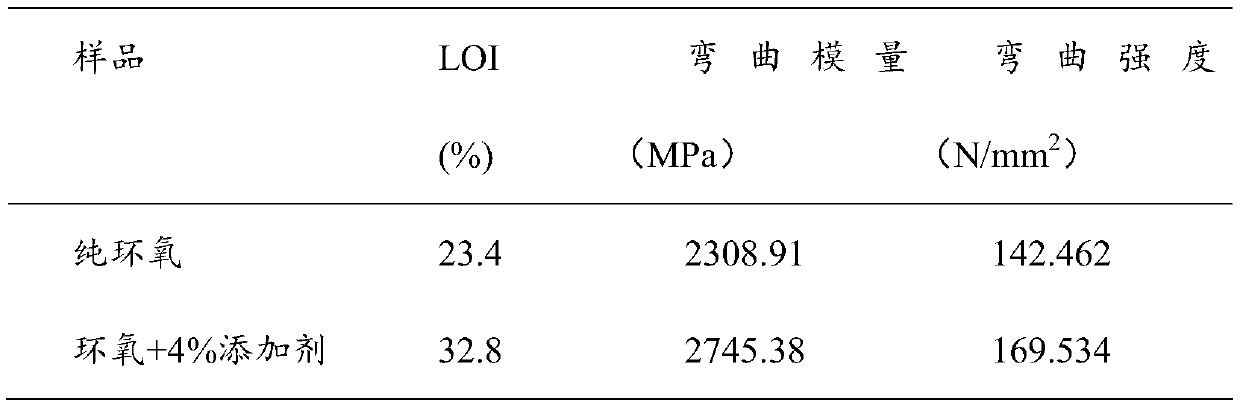
Method for synthesizing phosphorus-containing silicon-containing organic inorganic internal-hybridized activated monomer and application
A technology of active monomers and synthesis methods, which is applied in the fields of organic chemistry, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. It can solve the problems of complex methods and the inability to control the ratio of elements more accurately , to achieve the effect of simple synthesis method, precise and controllable element composition, and improved heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
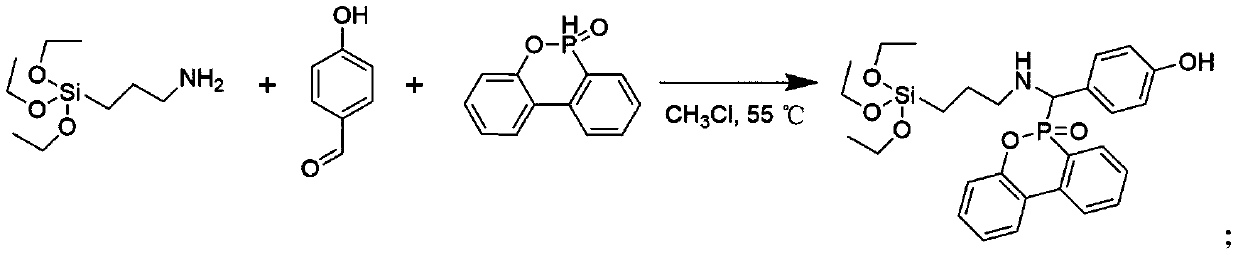
[0021] Add 11.68mL KH550 (50mmol), 6.7g 4-hydroxyphenyl formaldehyde (55mmol), 11.9g DOPO (55mmol) and 300mL chloroform into a 1000mL round bottom flask connected with a condenser, and stir the reaction at 50°C for 12h. After the reaction was completed, the reaction solution was concentrated to about 50 mL by rotary evaporation, and then slowly dropped into iced methanol for precipitation. It was filtered under reduced pressure, washed three times with methanol, and freeze-dried to obtain a white solid.
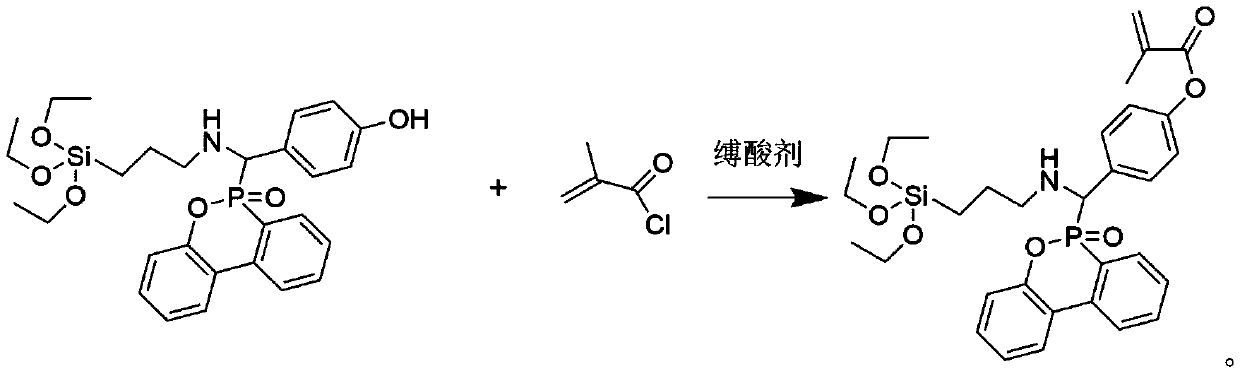
[0022] Add 27.1g (0.05mol) of previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of dichloromethane to a 250mL single-necked round-bottomed flask in sequence, place it at 5°C, and slowly 10.5 g (0.10 mol) of methacryloyl chloride was added dropwise, and stirring was continued for 8 hours after the dropwise addition was completed. After the reaction was completed, triethylamine hydrochloride was removed by filtration, and washed ...
Embodiment 2
[0024] Add 11.68mL KH550 (50mmol), 4.9g 4-hydroxyphenyl formaldehyde (40mmol), 11.9g DOPO (55mmol) and 300mL chloroform into a 1000mL round bottom flask connected with a condenser, and stir the reaction at 50°C for 12h. After the reaction was completed, the reaction solution was concentrated to about 50 mL by rotary evaporation, and then slowly dropped into iced methanol for precipitation. It was filtered under reduced pressure, washed three times with methanol, and freeze-dried to obtain a white solid.
[0025] Add 27.1g (0.05mol) of previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of dichloromethane to a 250mL single-necked round-bottomed flask in sequence, place it at 5°C, and slowly 10.5 g (0.10 mol) of methacryloyl chloride was added dropwise, and stirring was continued for 8 hours after the dropwise addition was completed. After the reaction was completed, triethylamine hydrochloride was removed by filtration, and washed ...
Embodiment 3
[0027] 11.68mL KH550 (50mmol), 4.9g 4-hydroxyphenyl formaldehyde (40mmol), 11.9g DOPO (55mmol) and 300mL ethanol were added to a 1000mL round bottom flask connected with a condenser, and the reaction was stirred at 50°C for 12h. After the reaction was completed, the reaction solution was concentrated to about 50 mL by rotary evaporation, and then slowly dropped into iced methanol for precipitation. It was filtered under reduced pressure, washed three times with methanol, and freeze-dried to obtain a white solid.
[0028] Add 27.1g (0.05mol) of previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of dichloromethane to a 250mL single-necked round-bottomed flask in sequence, place it at 5°C, and slowly 10.5 g (0.10 mol) of methacryloyl chloride was added dropwise, and stirring was continued for 8 hours after the dropwise addition was completed. After the reaction was completed, triethylamine hydrochloride was removed by filtration, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com