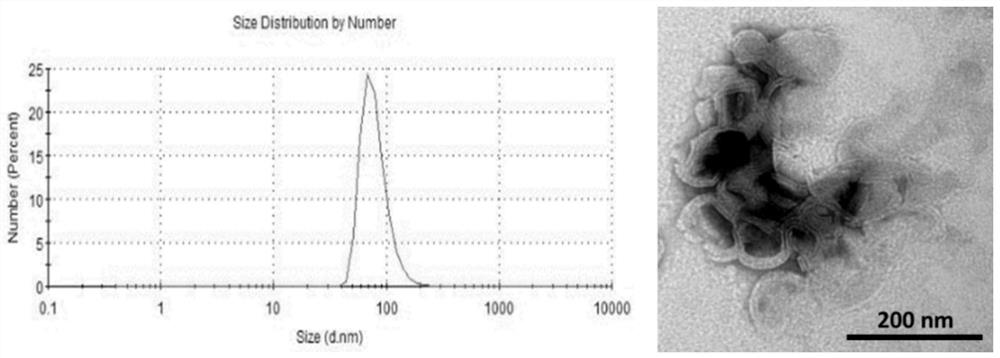
Extracellular Vesicle Isolation and Enrichment Method Based on Size Exclusion Chromatography and Ultrafiltration
A technology of size exclusion chromatography and vesicles, applied in the field of extracellular vesicle separation and enrichment, can solve the problems of easy blocking of filter membrane, loss of EVs, low concentration, etc., and achieve the effect of improving nucleic acid purity and eliminating nucleic acid pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 fluorescent liposome
[0039] 1. Preparation of reagent consumables
[0040] Constant pressure control extrusion device (Avestin LiposoFast Basicn) was purchased from Canada Avestin. Phosphatidylcholine, phosphatidylethanolamine, chloroform and methanol were purchased from Sigma-Aldrich. PBS was purchased from Solebol. Fluorescein DHPE was purchased from Thermo Fisher Scientific. Ultrafiltration tubes were purchased from millipore.
[0041] 2. Preparation of liposomes by constant pressure controlled extrusion
[0042] Use a 250 μL sealed glass syringe to add 100 μL chloroform into a 20 mL glass bottle; then use a 100 μL sealed glass syringe to add 30 μL methanol to the same glass bottle; then add 10 mg / mL phosphatidylcholine 216 μL, 10 mg / mL phosphatidylethanolamine 42 μL and 20 μL of 11 mg / mL cholesteryl ester were sequentially added to the above glass bottle; 0.2 mmol of Fluorescein DHPE was added; the organic solvents chloroform a...
Embodiment 2
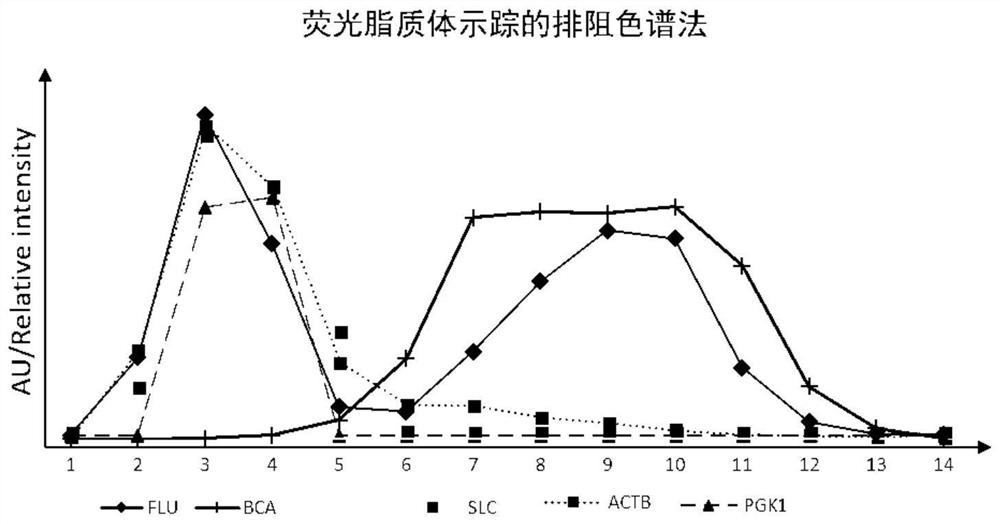
[0047] Example 2 Purification of EVs by exclusion method based on fluorescent liposome tracking
[0048] 1. Reagent preparation
[0049] Centrifuge commercial human plasma at 1300g for 15min, then transfer the supernatant to a new centrifuge tube and centrifuge at 3000g for 15min. The plasma supernatant was then filtered through a 0.8 μm filter to remove cytoplasmic debris. Take 0.5 mL of plasma supernatant, and add 2 μl of fluorescent liposome solution to it at a volume ratio of 250:1.
[0050] 2. Purification of plasma EVs by size exclusion chromatography
[0051] Pack 5 mL of Sepharose 2B packing into a 6 mL size exclusion column. After fully equilibrating with PBS buffer, add 0.5mL plasma supernatant containing fluorescent liposomes to the exclusion column. Then it was eluted with mobile phase, and 14 fractions were sequentially collected in a volume of 250 μL. The mobile phase used was PBS buffer, and the elution flow rate was about 200-500 μL / min.
[0052] 3. Compo...
Embodiment 3
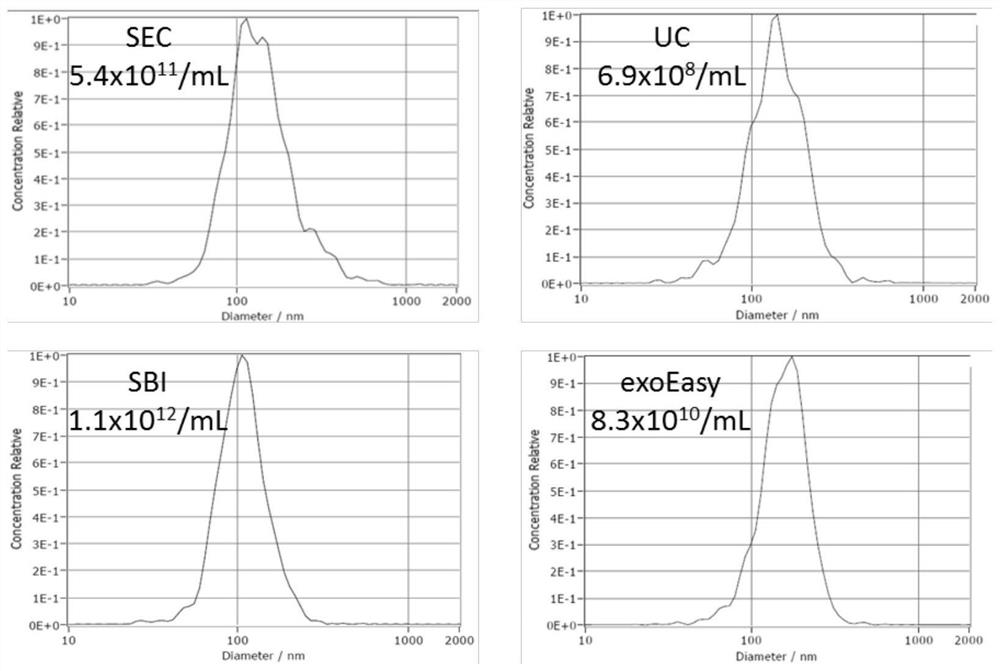
[0074] Example 3 Comparison of the EVs extraction method based on size exclusion chromatography and ultrafiltration with other methods
[0075] 1. Reagent preparation
[0076] Centrifuge commercial human plasma at 1300g for 15min, then transfer the supernatant to a new centrifuge tube and centrifuge at 3000g for 15min. Then the plasma supernatant was filtered with a 0.8 μm filter membrane to remove the cytoplasm and the residue was set aside.
[0077] 2. EVs extraction by size exclusion chromatography and ultrafiltration (SEC)
[0078] Take three 6ml size exclusion chromatography columns (containing 5mL Sepharose 2B filler). After equilibration, 0.5 mL of filtered plasma supernatant (total 1.5 mL of plasma) was added, and 2 μl of fluorescent liposome solution was added thereto at a volume ratio of 1000:4. Fourteen fractions were collected sequentially in 250 μL volumes. Referring to Experimental Example 2, the corresponding fractions were collected and mixed, then added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com