Polypeptide, polypeptide-modified lipid carrier and application of lipid carrier
A technology of lipid carrier and liposome, which is applied in the field of polypeptide-modified lipid carrier and polypeptide, can solve the problems of reducing phagocytosis of macrophages, low clinical value, and difficult production, so as to improve delivery in vivo, inhibit phagocytosis, The effect of reducing the distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The implementation mode of linking cholesterol to polypeptide is according to literature [Ingallinella P, Bianchi E, Ladwa NA, et al. Addition of a cholesterol group to an HIV-1 peptide fusion inhibits dramatically increases its antiviral potency. Proceedings of the National Academy of Sciences, 2009, 106( 14): 5801-5806.] Carry out, specifically, obtain polypeptide C by solid-phase synthesis D GK D L D E. D I D V D T D K D GE D R D S D L D E. D T D V D E. D C D T D Y D N D G. Dissolve 100 mg of cholesterol (injection grade, purchased from Everett Company) and 40 mg of bromoacetic acid (purity greater than 99%, purchased from Adamas) in 1500 μL of anhydrous dichloromethane (purchased from Sigma Company), and add 44 μL of N, N'-diisopropylcarbodiimide (98%+, purchased from Adamas) and 1.5mg 4-dimethylaminopyridine (99%, purchased from Adamas) were used as catalysts, stirred at 400rpm at room temperature for 48h, then reduced Dry under pressure to obtai...
Embodiment 2
[0041] Both L-type "Self" peptide (LMS) and D-type "Self" peptide (DMS) are synthesized by solid-phase synthesis, wherein the specific sequence of LMS is as follows: GNYTCEVTELSREGKTVIELK (as shown in SEQ ID NO.1), and in the amino The segment is synthesized by solid phase [Cao L, Fischer A, Bornscheuer U T, et al. Lipase-Catalyzed Solid Phase Synthesis of Sugar Fatty Acid Esters. Biocatalysis, 1996, 14(4): 15.] Adding palmitic acid, the specific sequence of DMS is as follows : GK D L D E. D I D V D T D K D GE D R D S D L D E. D T D V D E. D C D T D Y D N D G, and add palmitic acid at the amino segment by solid-phase synthesis.
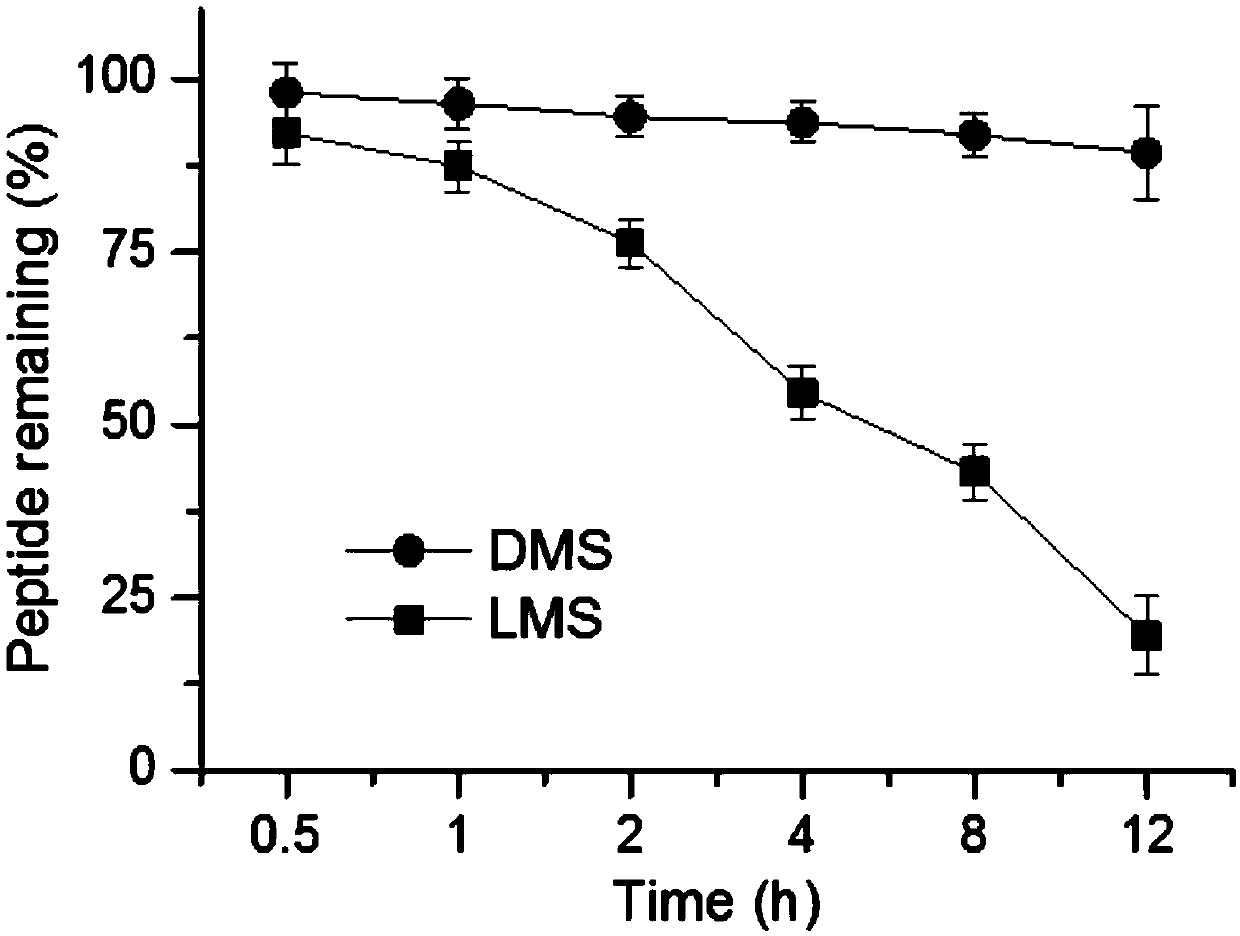
[0042] The two configurations of polypeptides were prepared as 5 mg / ml solutions in PBS, and mouse serum (Abbkine) was added to RPMI 1640 to obtain solution A containing 25% (volume ratio) of mouse serum. Add the peptide solution to solution A to a final peptide concentration of 100 μg / ml. Incubate at 37°C for 0.5, 1, 2, 4, 8, and ...
Embodiment 3
[0044]Polypeptide solution (1 mg / ml) was prepared in methanol, egg yolk lecithin (PL-100m, Ivet) solution (28 mg / ml) was prepared in ethanol, cholesterol (injection grade, purchased from Ivet) was prepared in chloroform Special) solution (12mg / ml). The phospholipid, cholesterol and polypeptide solutions were mixed at 500:250:40 (volume ratio) to obtain about 2ml of mixed solution. Evaporate to dryness under reduced pressure to obtain a phospholipid film, and remove a small amount of organic solvent with nitrogen gas. PBS solution (pH = 7.2) was added to the film and shaken in a constant temperature water bath at 37° C. to obtain multilamellar liposomes. The liposomes were respectively passed through liposome extruders containing 400nm, 200nm and 100nm polycarbonate membranes for 20 times each to obtain the desired polypeptide liposomes, which were stored for future use.
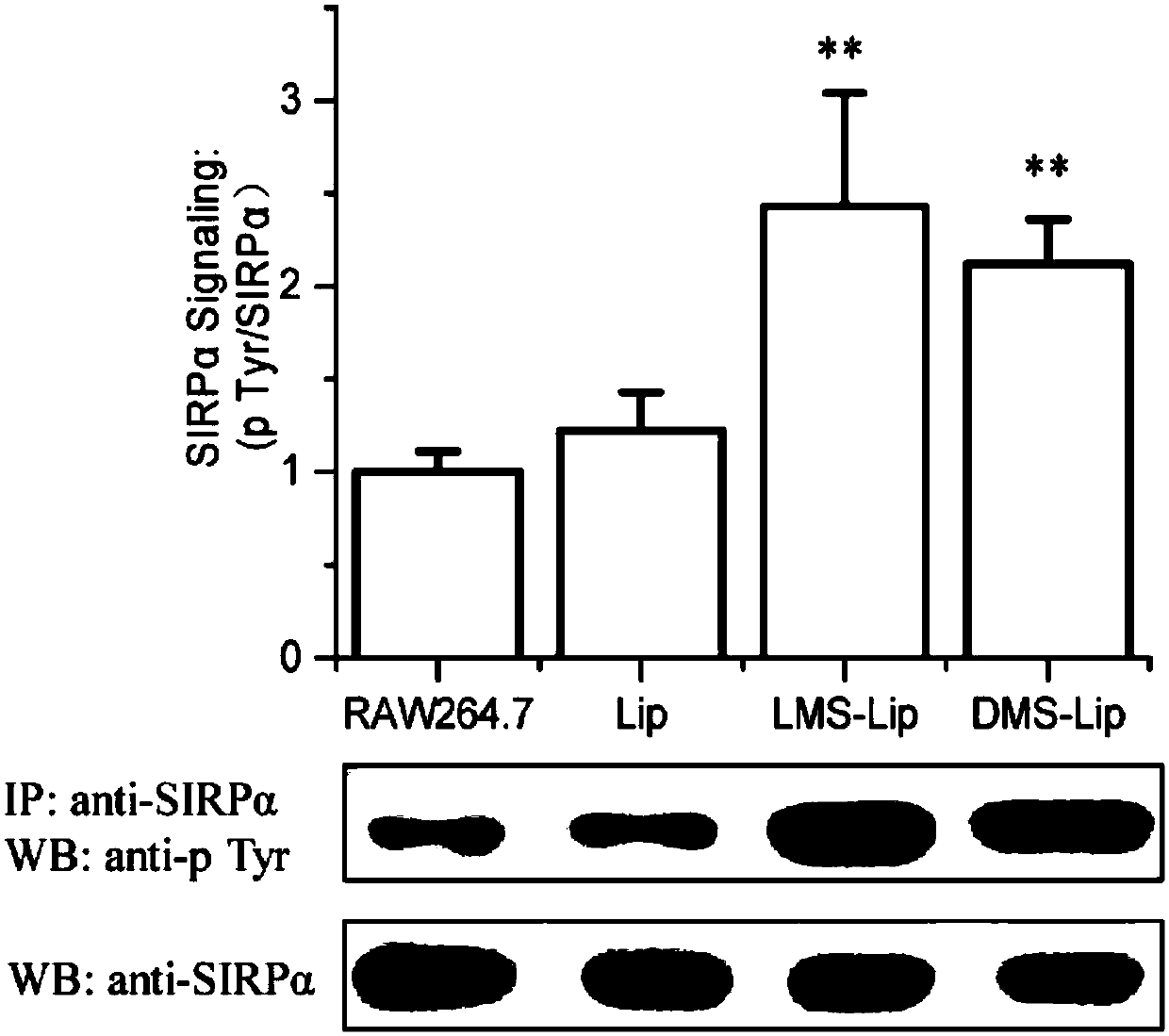
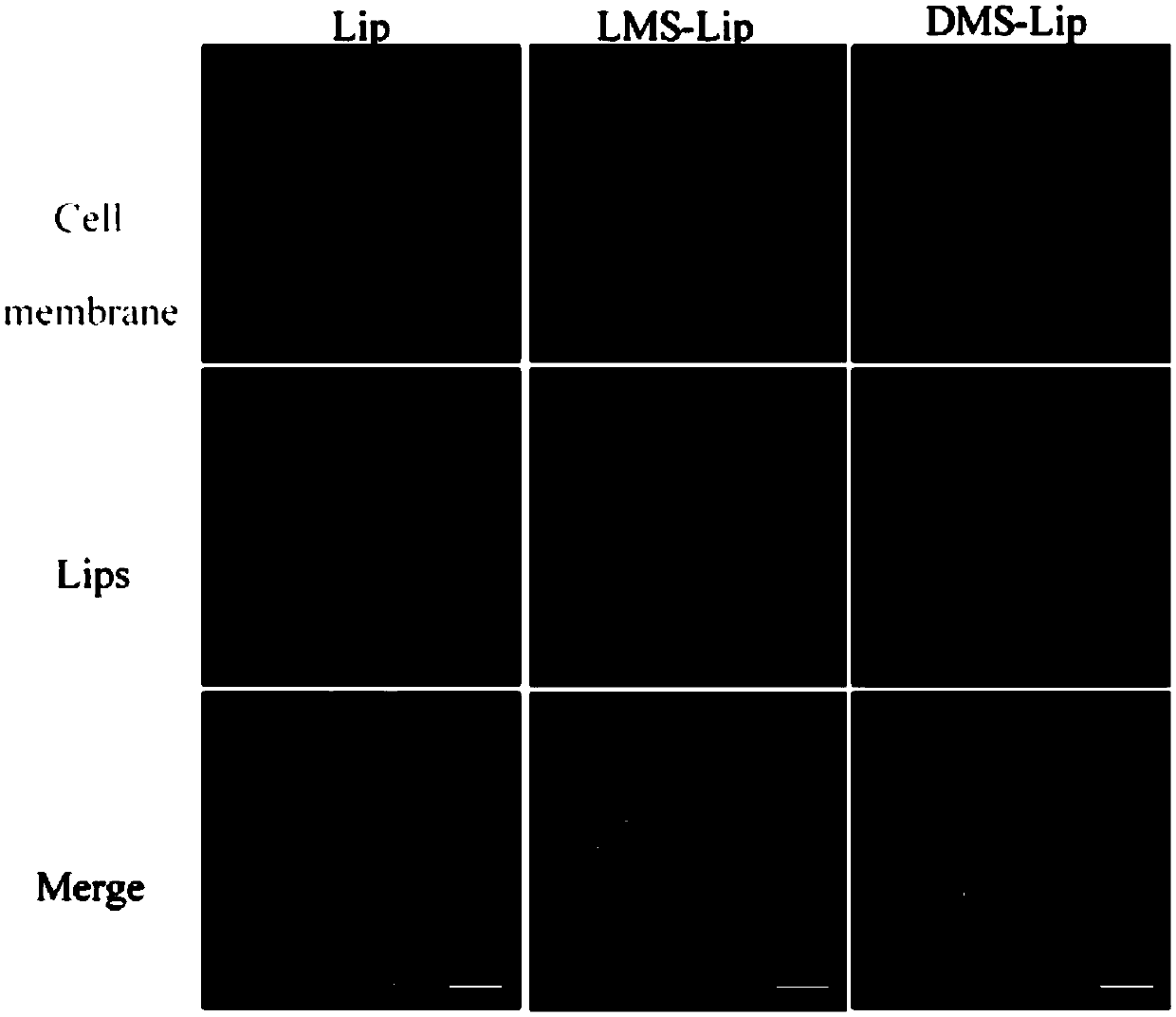
[0045] RAW264.7 cells in the logarithmic growth phase (purchased from the Shanghai Cell Bank of the Chin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com