Preparation method of oseltamivir phosphate
A technology of oseltamivir phosphate and temperature control, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, and the preparation of cyanide reactions, etc., which can solve the research and development limitations of oseltamivir phosphate generic drugs and the inability of the original research and preparation route to be effective. Substitute, process cost and industrialization transformation competition and other issues, to achieve the effect of high total yield, mild process conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
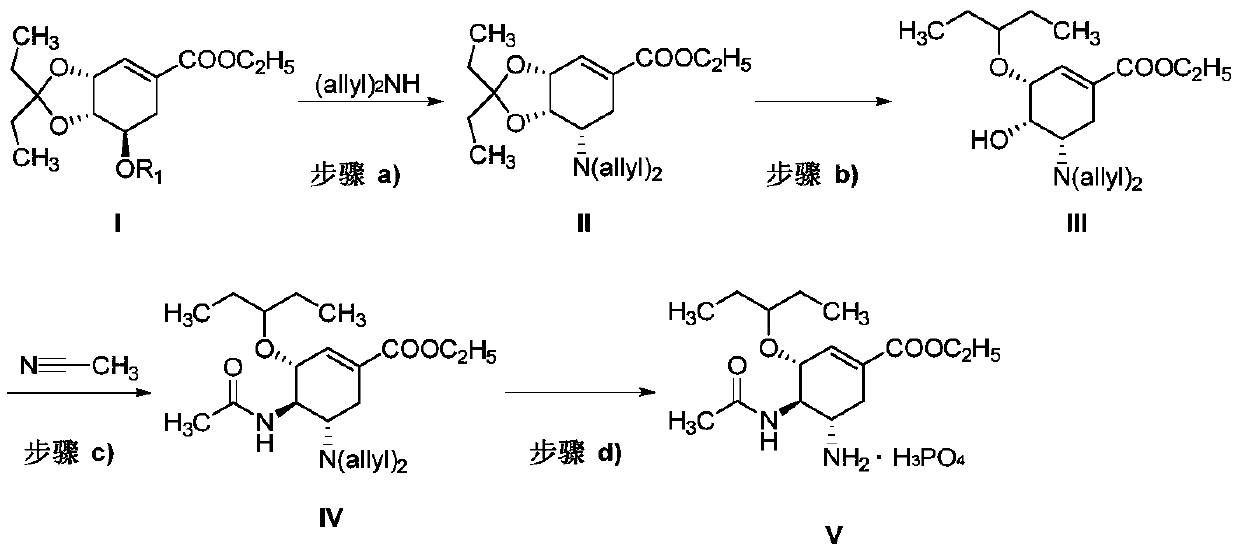
[0044] Preparation of Example 1 Compound II
[0045]
[0046] Compound I(R 1 Methanesulfonyl, 500.0g, 1.44mol, 1.0eq.) was dissolved in 5L N,N-dimethylformamide (DMF), and cesium carbonate (1407.4g, 4.32mol, 3.0eq.) was added under stirring, and then Diallylamine ((allyl) 2 NH; 168.2g, 1.73mol, 1.2eq.), the temperature was raised to 90-100°C for 8 hours, and the reaction was complete as monitored by TLC. The reaction solution was cooled to room temperature and filtered. Add 20L of water to the filtrate for dilution, then add 5L of ethyl acetate to stir and separate the liquids, the organic phase is washed with 5% aqueous sodium chloride, then dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain 479.2g of orange oil formula II (theoretical The value is 503.3g), and the yield is 95%.
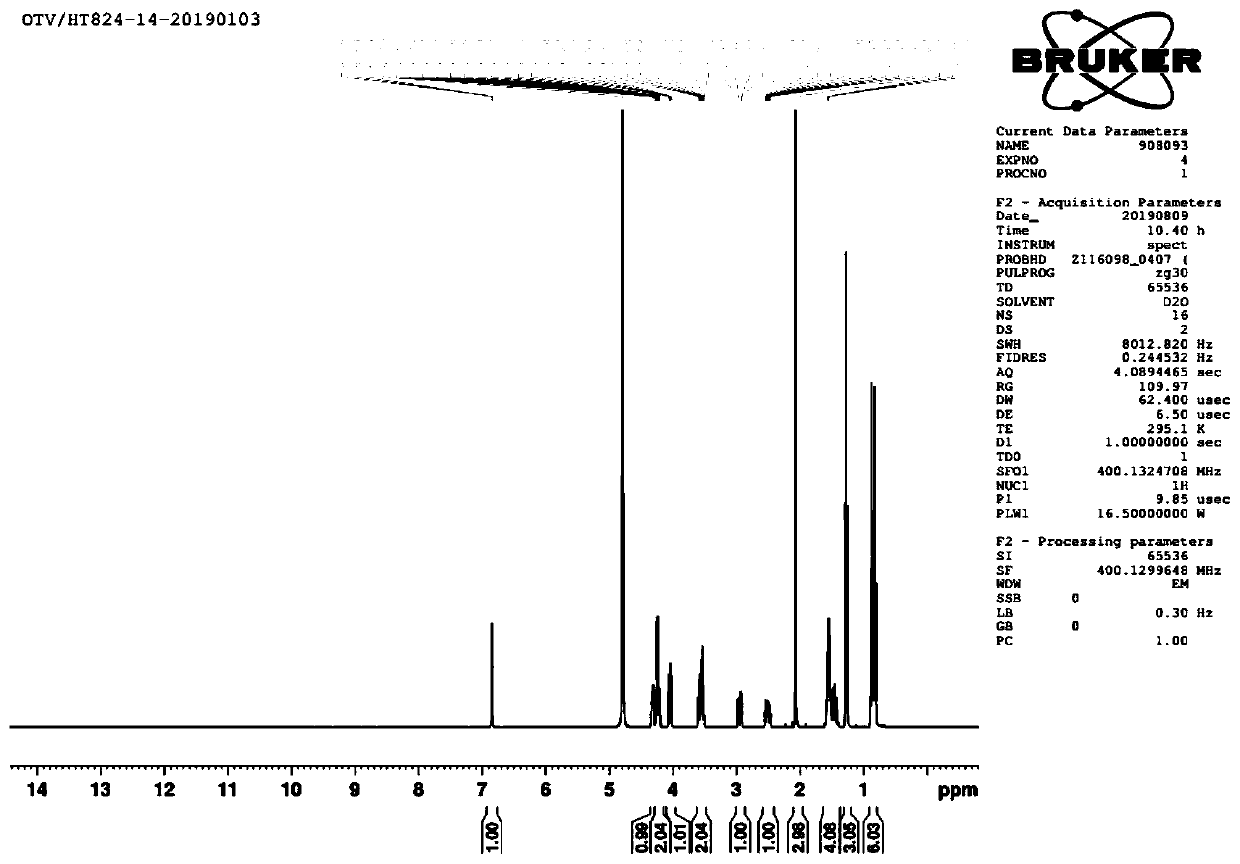
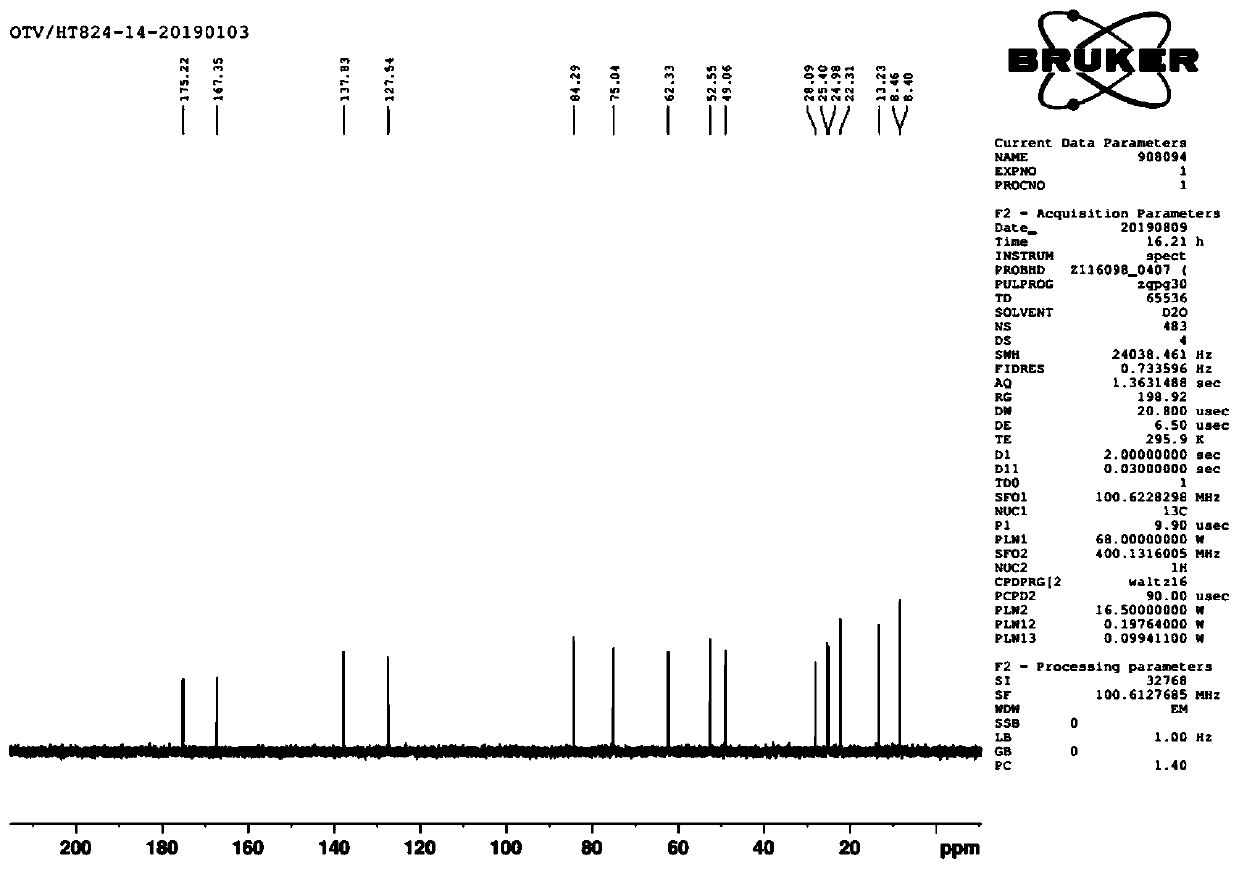
[0047] 1 H NMR (CDCl 3 ,400MHz)δ6.93(m,1H),5.77-5.66(m,2H),5.18(d,2H,J=17Hz),5.06(d,2H,J=10Hz),4.22(m,2H), 4.09(m,1H),3.90(m,1H),3.39(m,1H),3.29(m,2H),2.93(...
Embodiment 2
[0050] Embodiment 2 Compound III Preparation
[0051]
[0052]Compound II (479.2g, 1.37mol, 1.0eq.) was dissolved in 4.5L of dichloromethane, the temperature was lowered to -40~-35°C under the protection of nitrogen, and triethylsilane (223.3g, 1.92mol, 1.4eq.), titanium tetrachloride (299.7g, 1.58mol, 1.15eq.) were dissolved in 1.5L dichloromethane solution, and reacted for 2 hours under temperature control and stirring, and the reaction was complete as monitored by TLC. 6L of water was added to the reaction solution with stirring, and the temperature was slowly raised to 0°C for liquid separation. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and 5% aqueous sodium chloride solution, then dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 418.9 g (theoretical value: 481.5 g) of orange oily substance formula III, with a yield of 87% .
[0053] 1 H NMR (CDCl 3 ,400MHz)δ6.73(m,1H),5.78-5.66...
Embodiment 3
[0056] Embodiment 3 Compound IV Preparation
[0057]
[0058] Compound III (418.9g, 1.19mol, 1.0eq.) was dissolved in 1L of acetonitrile, cooled to 0°C, and a mixed solution of sulfuric acid and acetic acid (sulfuric acid: 50mL; acetic acid: 100mL) was added dropwise, and the temperature was slowly raised to 20 °C for 1 hour. TLC monitored the completion of the reaction. The reaction solution was slowly added dropwise into water at 0-5° C., then extracted twice with 2 L of ethyl acetate, and the organic phases were combined. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and 5% aqueous sodium chloride solution, then dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 415.7 g (theoretical value 467.1 g) of orange oily substance formula IV, with a yield of 90% .
[0059] 1 H NMR (CDCl 3 ,400MHz)δ6.73(m,1H),5.78-5.66(m,2H),5.36(d,1H),5.17(d,2H,J=16.5Hz),5.08(d,2H,J=10Hz) ,4.22(m,2H),4.09(m,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com